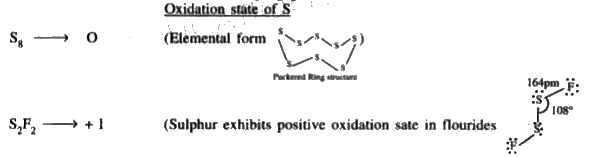
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VIA GROUP ELEMENTS-PRACTICE SHEET - 5 ( Integer answer type Questions)
- When This sulphate ion is oxidized by iodinc, new product 'X' is forme...
Text Solution
|
- Find the the number of ions having S-S bond from the following S(2)...
Text Solution
|
- How many of the following is not oxidised by O(3) ? KI, FeSO(4), KMnO(...
Text Solution
|
- The difference in the oxidation state of the two types of 'S' atoms in...
Text Solution
|
- The oxidation state of the product when 'KBr' and 'KBrO(3)' react in a...
Text Solution
|
- When F(2) react with H(2)S a product (x) of sulphur is formed the diff...
Text Solution
|