Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (SINGLE OR MORE THAN ONE OPTION QUESTIONS)|16 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (LINKED COMPREHENSION TYPE QUESTIONS)|6 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise LEVEL-II (LECTURE SHEET) (EXERCISE-III)(MATCH OF FOLLOWING QUESTIONS)|9 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 VideosD - BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|50 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-D & F BLOCK ELEMENTS-LEVEL-II (LECTURE SHEET) (EXERCISE-IV)(INTEGER ANSWER TYPE QUESTIONS)
- A metal ion from the first transition has a magnetic moment of 3.87 B....
Text Solution
|
- The brown ring complex is formulated as [Fe(H2O)(5)NO^(+)]SO4 The Oxi...
Text Solution
|
- How many moles of KMnO4 are required to oxidise 10 moles of ferrous ox...
Text Solution
|
- When potassium dichromate is heated to a white heat, it decomposes to ...
Text Solution
|
- How many electrons are involved in reduction of KMnO4 in basic reducti...
Text Solution
|
- How many unpaired electrons are present in the central metal ion [CoCl...
Text Solution
|
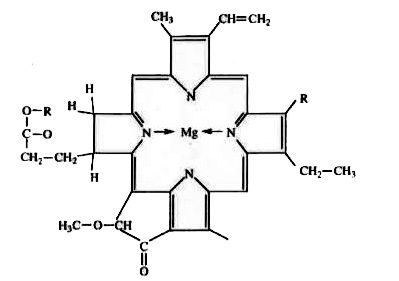
- Number of dative bonds around Mg^(2+) ion in chlorophyll is
Text Solution
|
- Maximum number of oxygen molecules bond around a heamoglobin is (are)
Text Solution
|
- Number of Cr-O sigma bonds in dichromate in Cr(2)O(7)^(2-) is
Text Solution
|
- Permanganate ion oxidises manganous ion to manganese dioxide in presen...
Text Solution
|