A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
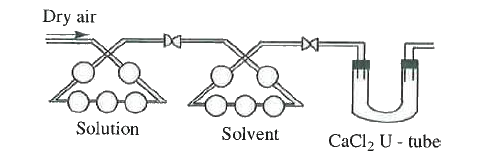
- A current of dry air is passed through the above apparatus containing ...
Text Solution
|
- Dry air was passed successively through a solution of 5g of a solute i...
Text Solution
|
- Dry air is passed through a solution containing 10 g of the solute in ...
Text Solution
|
- A current of dry air was passed first through a series of bulbs contai...
Text Solution
|
- A curent of dry air was bubbled through in a bulb containing 26.66g of...
Text Solution
|
- Lowering of vapour pressure is determined by Ostward and Walner dynami...
Text Solution
|
- A stream of dry air was passed through a bulb containing a solution of...
Text Solution
|
- A Current of dry air was first passed through the bulb containing solu...
Text Solution
|
- A Current of dry air was first passed through the bulb containing solu...
Text Solution
|