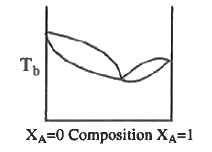
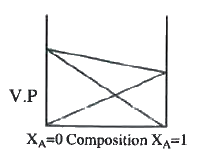
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two liquids A and B with vapour pressures 400mm Hg and 600mm Hg respec...
Text Solution
|
- Two liquids A and B form an ideal soluton. The vapour pressure of pure...
Text Solution
|
- The vapour pressure of pure liquids A and B is 450 and 700mm Hg , resp...
Text Solution
|
- The vapour pressures of pure liquids A B are 450 mm and 700 mm of Hg r...
Text Solution
|
- The vapour pressure of pure liquids A and B is 450 and 700mm Hg, resp...
Text Solution
|
- The vapour pressure of pure liquids A and B are 450 and 700 mm Hg resp...
Text Solution
|
- The vapour pressure of pure liquids A and B is 450 and 700mm Hg, resp...
Text Solution
|
- The vapour pressure of pure liquids A and B are 450 and 700 mm Hg resp...
Text Solution
|
- The vapour pressure of a mixure of 1 mole of liquid 'A' and 3 mole of ...
Text Solution
|