A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
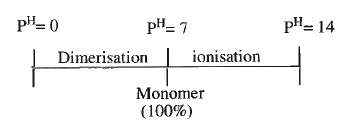
- An organic compound (Mol.wt=100) exist in "Monomeric from" in its neut...
Text Solution
|
- During the electrolysis of 50% H(2)SO(4), the p^(H) of the solution
Text Solution
|
- For an acid buffer solution the P^(H) is 3. The p^(H) can be increased...
Text Solution
|
- Equal volumes of two solutions with P^(H) = 3 and P^(H) = 11 are mixed...
Text Solution
|
- The P^(H) of a solution is 6 . Its H^(+) concentrations is decreased b...
Text Solution
|
- During the electrolysis of 50% H(2)SO(4), the p^(H) of the solution
Text Solution
|
- The [H^+] of a solution is 10^(-8) . Its p^(OH) is
Text Solution
|
- The [H^+] of a solution is 10^(-8) . Its p^(OH) is
Text Solution
|
- The P^(H) of a solution is 3.602. Its H^(+) ion concentration is
Text Solution
|