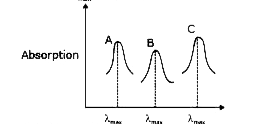Similar Questions
Explore conceptually related problems
Recommended Questions
- Simplified absorption spectra of three complexes ((i), (ii) and (iii))...
Text Solution
|
- The value of lim(n rarr oo)(nC(3)-nP(3))/(n^(3)) is equal to (I)-(5)/(...
Text Solution
|
- If lim(x rarr3)(x^(n)-3^(n))/(x-3)=108,n in N then value of n is (i)5(...
Text Solution
|
- The absorption maxima of several octahedral complex ions are as follow...
Text Solution
|
- The magnetic moment of complex given below are in the order: (I) [Ni(C...
Text Solution
|
- (i) [Co ( H(2) O)(6) ]^(n-6) (ii) [PtCl(2) BrF]^(2-) m geometrical iso...
Text Solution
|
- Simplified absorption spectra of three complexes ((i), (ii) and (iii))...
Text Solution
|
- The magnetic moment of complex given below are in the order: (I) [Ni(C...
Text Solution
|
- The absorption maxima of several octahedral complex ions are as follow...
Text Solution
|