Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-ELECTROCHEMISTRY-All Questions
- Value of standard electrode potential for the oxidation of Cl^(-) ions...
Text Solution
|
- What is electrode potential?
Text Solution
|
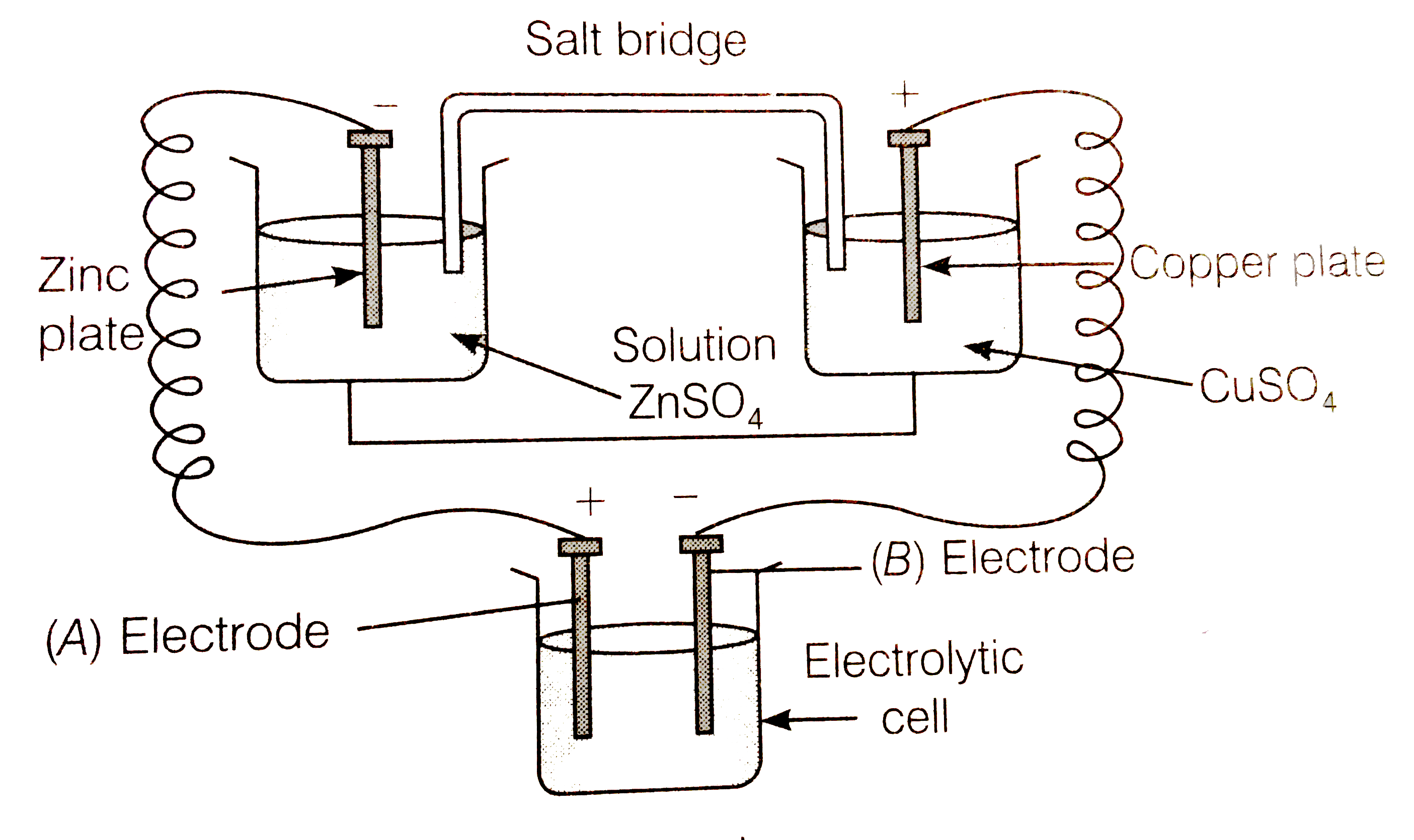
- Consider the following diagram in which an electrochemical cell is cou...
Text Solution
|
- Why is alternating current used for measuring resistance of an electro...
Text Solution
|
- A galvanic cell has an electrical potential of 1.1V. If an opposing p...
Text Solution
|
- How will the pH of brine (aq NaCl solution) be affected when it is ele...
Text Solution
|
- Unlike dry cell, the mercury cell has a constant cell potential throug...
Text Solution
|
- Solutions of two electrolytes A and B are diluted. The Lambda(m) of 'B...
Text Solution
|
- When acidulated water (dil. H(2)SO(4) solution) is electrolysed, will ...
Text Solution
|
- In an aqueous solution how does specific conductivity of electrolytes ...
Text Solution
|
- Which reference electrode is used to measure the electrode potential o...
Text Solution
|
- Consider a cell given below. Cu|Cu^(2+)||Cl^(-)|Cl(2).Pt Write the...
Text Solution
|
- Write the Nernst equation for the cell reaction in the Daniel cell. H...
Text Solution
|
- What advantage do the fuel cells have over primary and secondary batte...
Text Solution
|
- Write the cell reaction of a lead storage battery when it is discharge...
Text Solution
|
- Why on dilution the Lambda(m) of CH(3)COOH increases drastically, whil...
Text Solution
|
- Match the terms given in column I with the units given in column II.
Text Solution
|
- Match the terms given in Column I with the items given in Column II. (...
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|