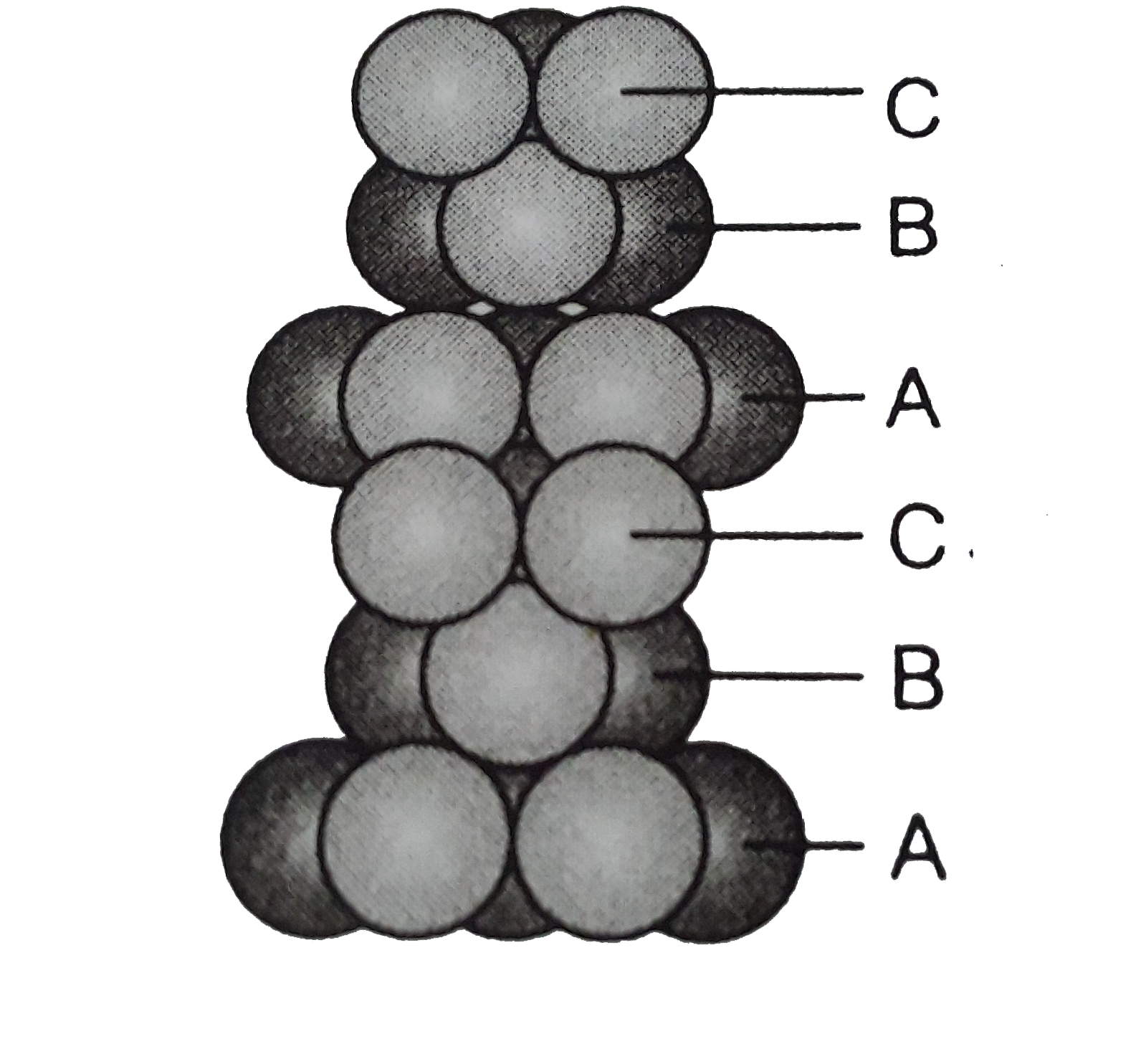
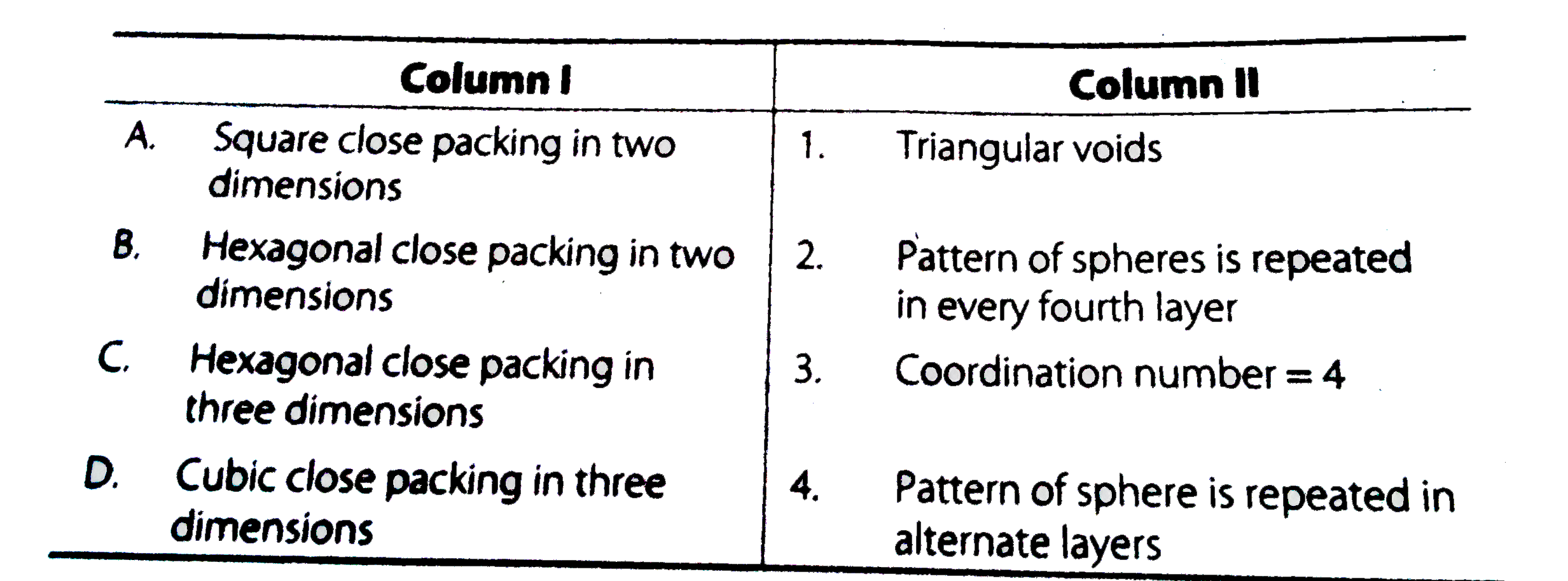Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
NCERT EXEMPLAR ENGLISH|Exercise matching the columns|1 VideosSOLID STATE
NCERT EXEMPLAR ENGLISH|Exercise long answer type questions|4 VideosSOLID STATE
NCERT EXEMPLAR ENGLISH|Exercise long answer type questions|4 VideosPOLYMER
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Question|5 VideosSOLUTIONS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|8 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-SOLID STATE-short answer type Questions
- Why are liquids and gases categorised as fluids ?
Text Solution
|
- Why are solids incompressible ?
Text Solution
|
- Inspite of long range order in the arrangement of particles why...
Text Solution
|
- Why common salt (NaCl) sometimes appear yellow?
Text Solution
|
- why is Fe0(s) not formed in stoichiometric compostion ?
Text Solution
|
- why does white Zn0(s) becomes yellow upon heating ?
Text Solution
|
- why does the electrical conductivity of semiconductors increase ...
Text Solution
|
- Explain why does conductivity of germainum crystals increase on ...
Text Solution
|
- A compound formed by two elements M and N. Element N forms ccp and ato...
Text Solution
|
- Under which situations can an amorphous substance change to cryst...
Text Solution
|
- match the type of unit cell given column I with the features iv...
Text Solution
|
- match the types of defect given in column I with the statement...
Text Solution
|
- match the items given in column I with the items given in colum...
Text Solution
|
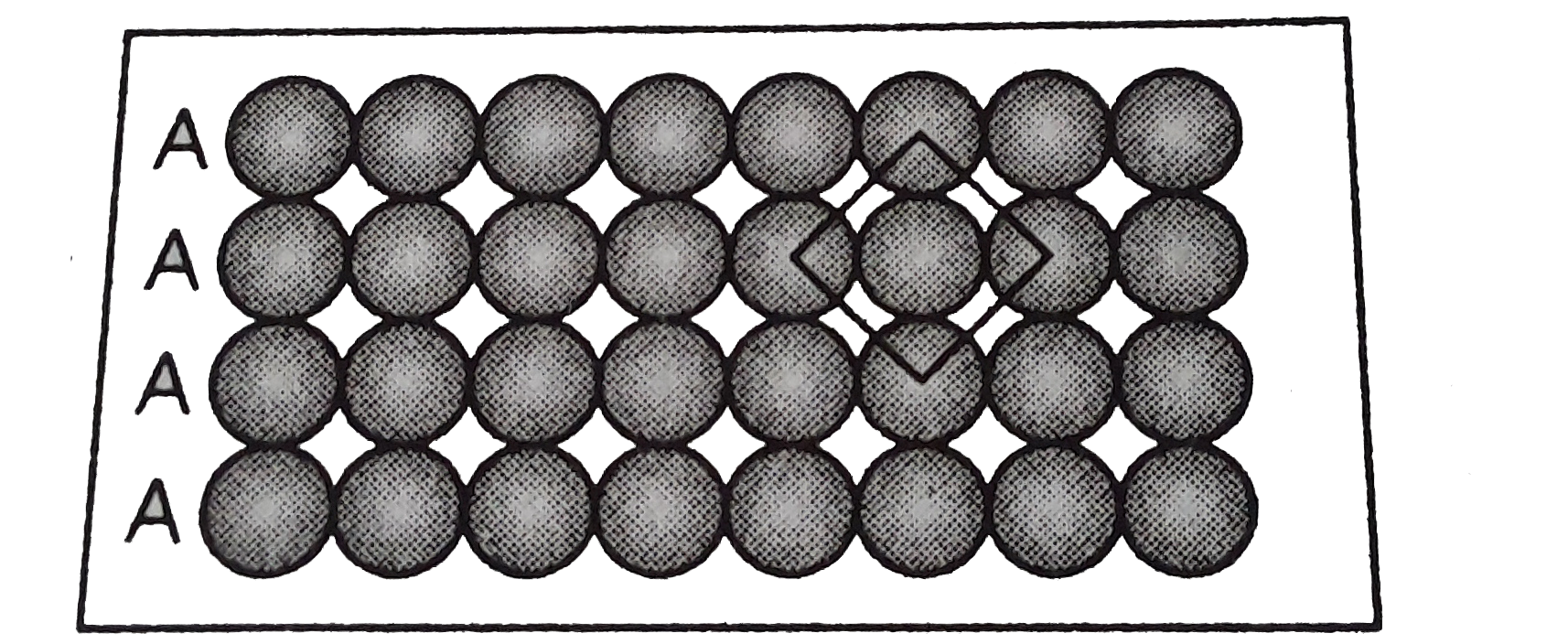
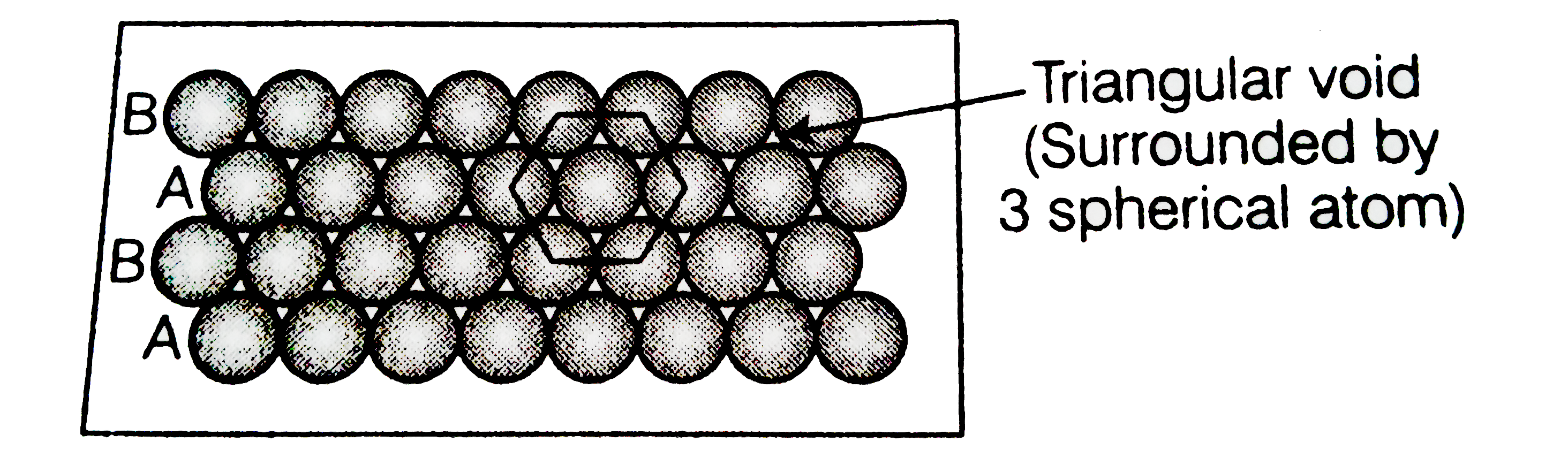
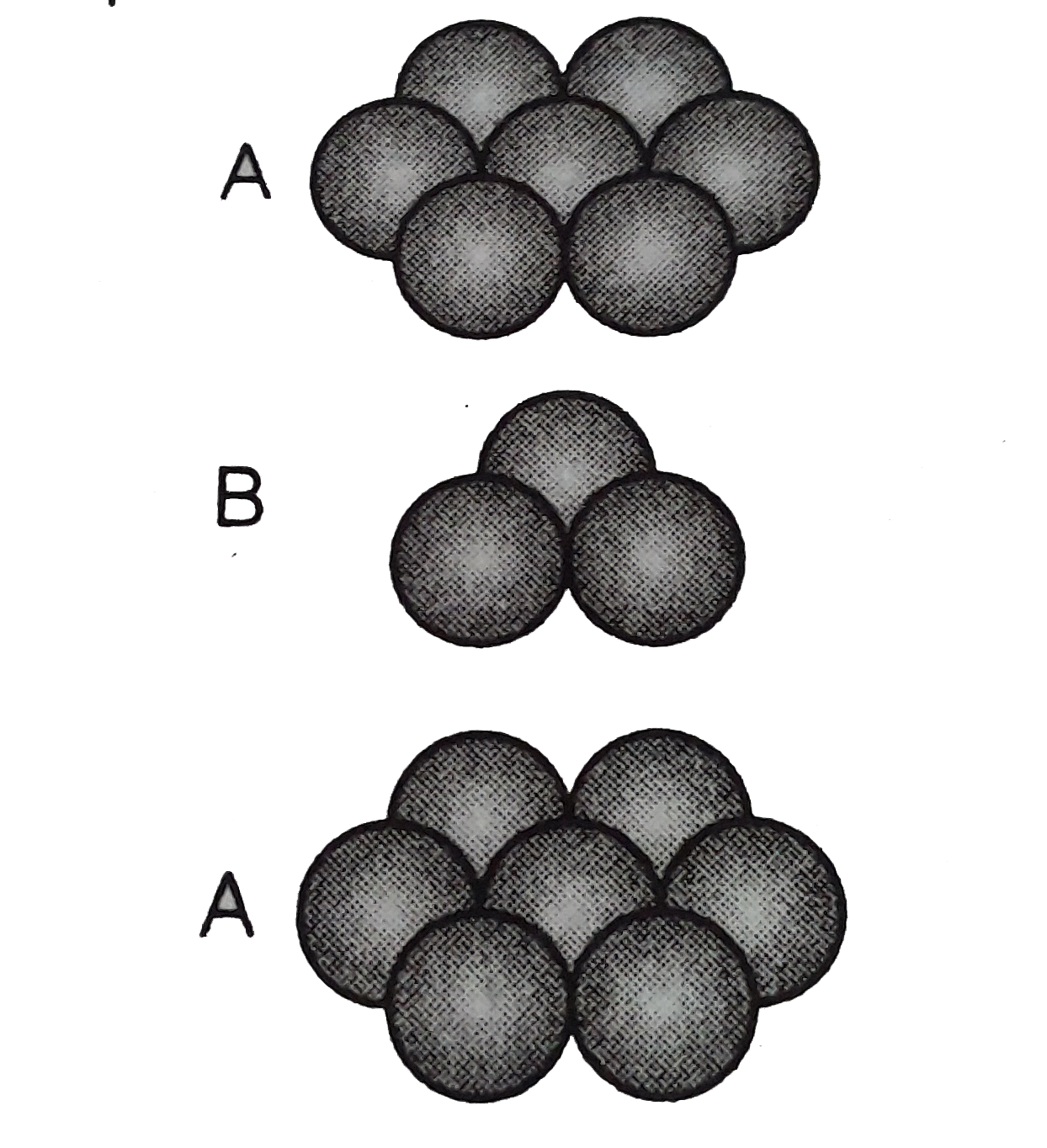
- Match the type of packing given in column I with the iterms give...
Text Solution
|
- Assertion :- (A) The total number of atoms present in a simple c...
Text Solution
|
- Assertion :- (A) Graphite is good conductor of electricity however ...
Text Solution
|
- Assertion :- (A) total number of octahedral voids present in ...
Text Solution
|
- Assertion :- (A) the paking efficiency is maximum for the fcc stru...
Text Solution
|
- Assertion :-(A) semiconductors are solids with conductivites in t...
Text Solution
|