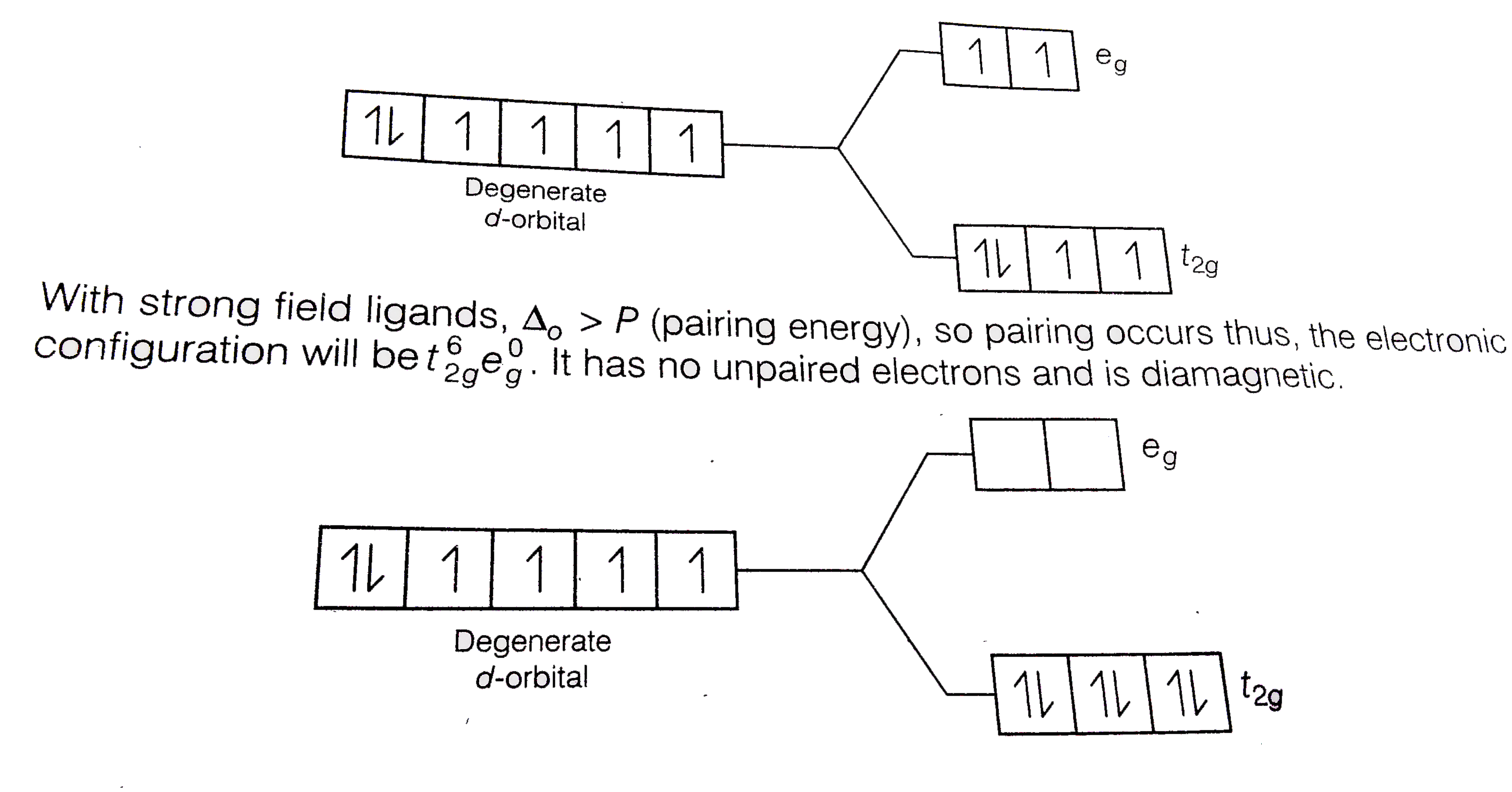Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Maching The Columns|5 VideosCOORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Assertion and Reason|5 VideosCOORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise MCQs More than one options|9 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Qns|4 VideosD AND F-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Question|20 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-COORDINATION COMPOUNDS-Short Answer Type
- Arrange the following complexes in the increasing order of conductivit...
Text Solution
|
- A coordination compound CrCI(3).4H(2)O precipitates AgCI when treated ...
Text Solution
|
- A complex of the type [M(A A)(2)X(2)] is known to the optically active...
Text Solution
|
- Magnetic moment of [MnCl(4)]^(2-) is 5.92 BM. Explain giving reason pr...
Text Solution
|
- On the basis of crystal field theory explain why Co(III) Forms paramag...
Text Solution
|
- Why are low spin tetrahedral complexes not formed ?
Text Solution
|
- Give the electronic configuration of the following complexes on the ba...
Text Solution
|
- Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM w...
Text Solution
|
- Arrange following complex ions in increasing order of crystal field sp...
Text Solution
|
- Why do compounds having similar geometry have different magnetic momen...
Text Solution
|
- CuSO(4).5H(2)O is blue in colour while CuSO(4) is colourless. Why ?
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attched to cen...
Text Solution
|