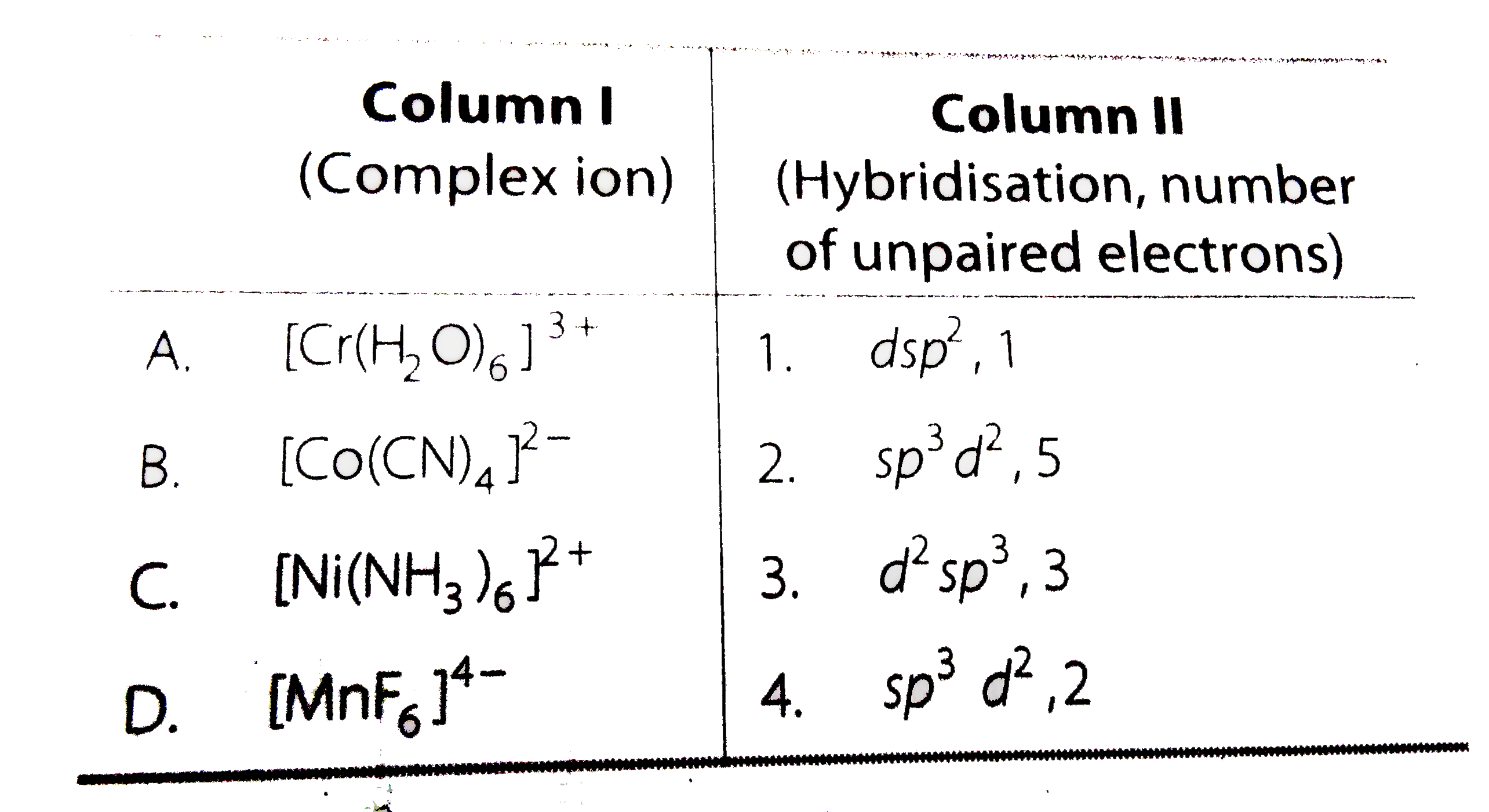
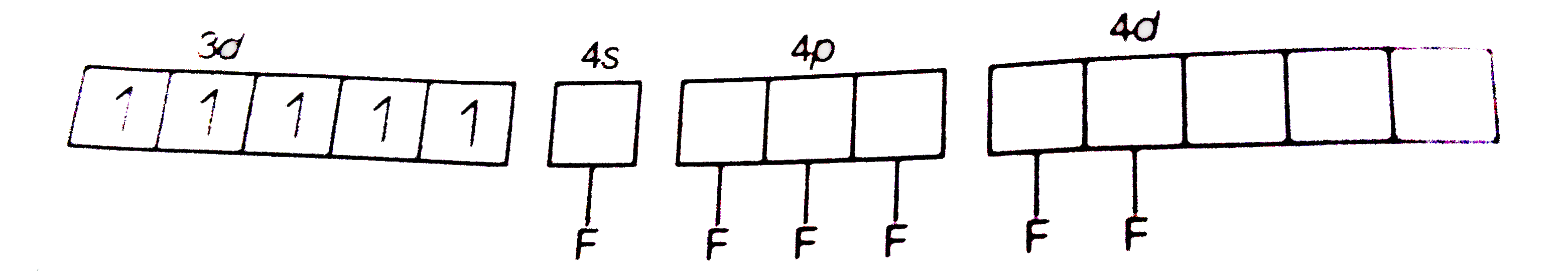
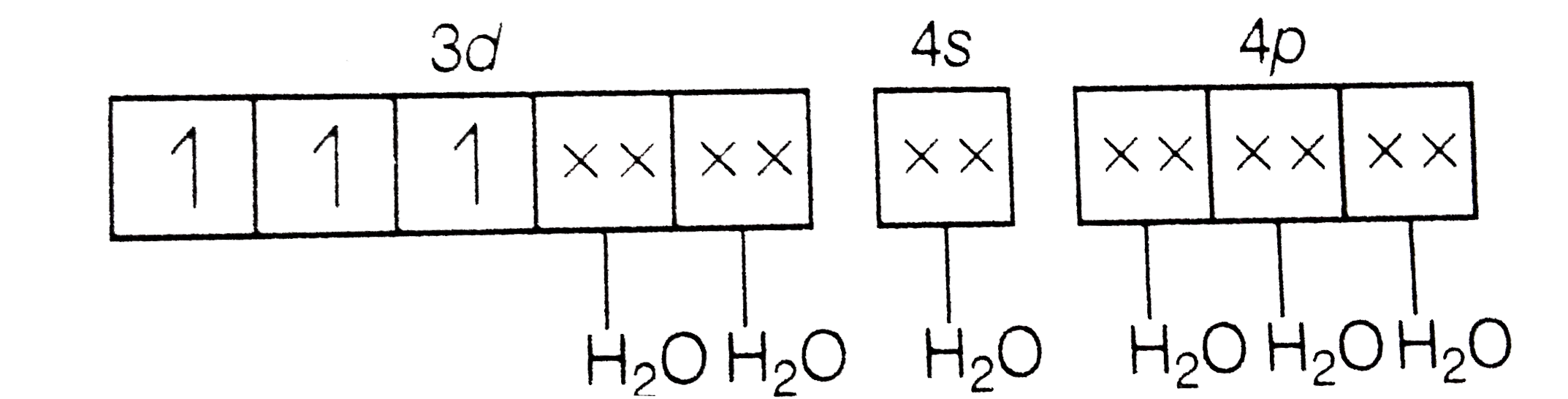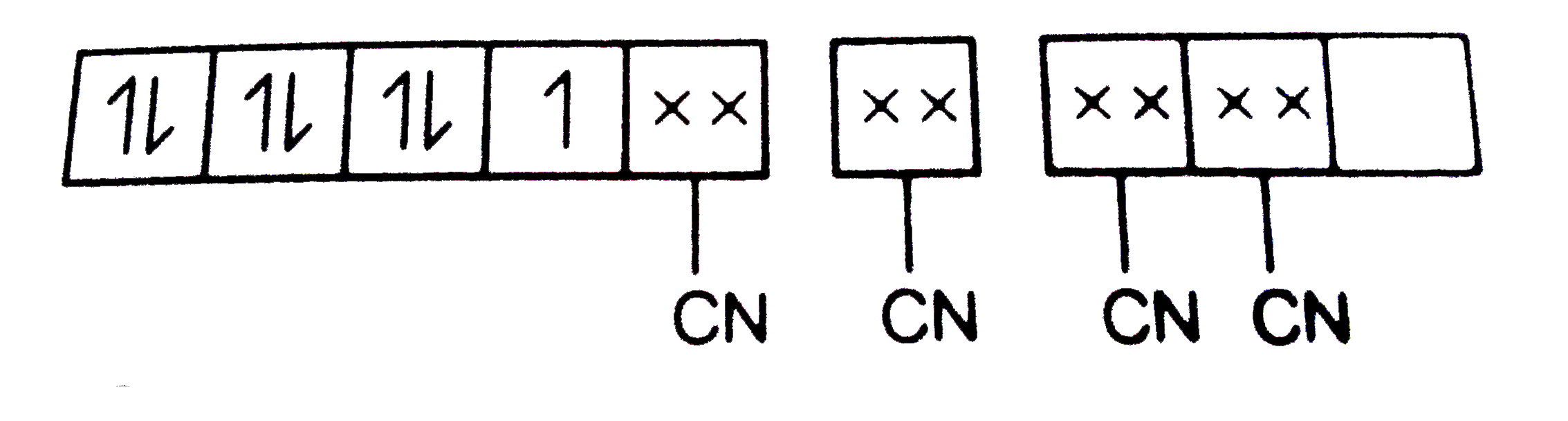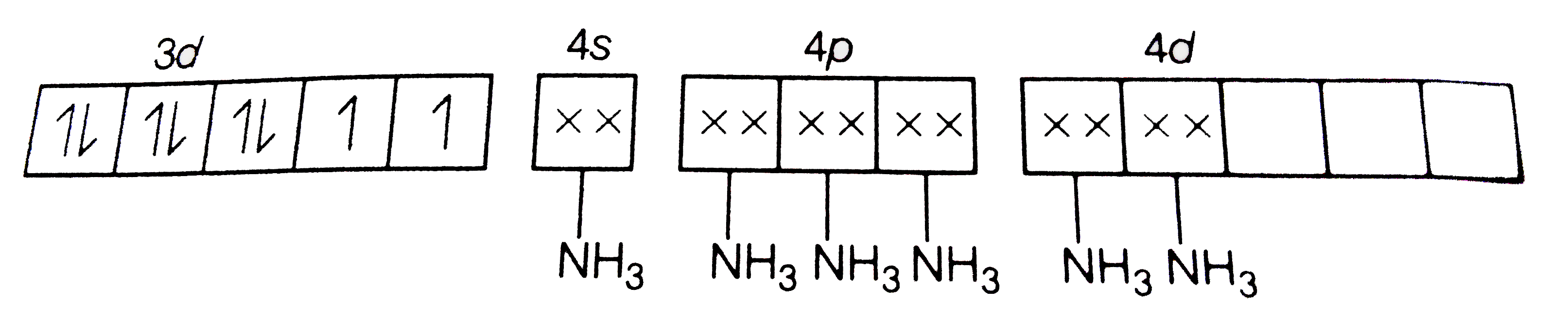A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Assertion and Reason|5 VideosCOORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Question|5 VideosCOORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type|12 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Qns|4 VideosD AND F-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Question|20 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-COORDINATION COMPOUNDS-Maching The Columns
- Match the complex ions given in column I with the colours given in col...
Text Solution
|
- Match the coordination compounds given in column I with the central me...
Text Solution
|
- Match the complex ions given in column I with the hybridisation and nu...
Text Solution
|
- Match the complex species given in column I with the possible isomeris...
Text Solution
|
- Match the compounds given In column I with oxidation state of cabalt p...
Text Solution
|