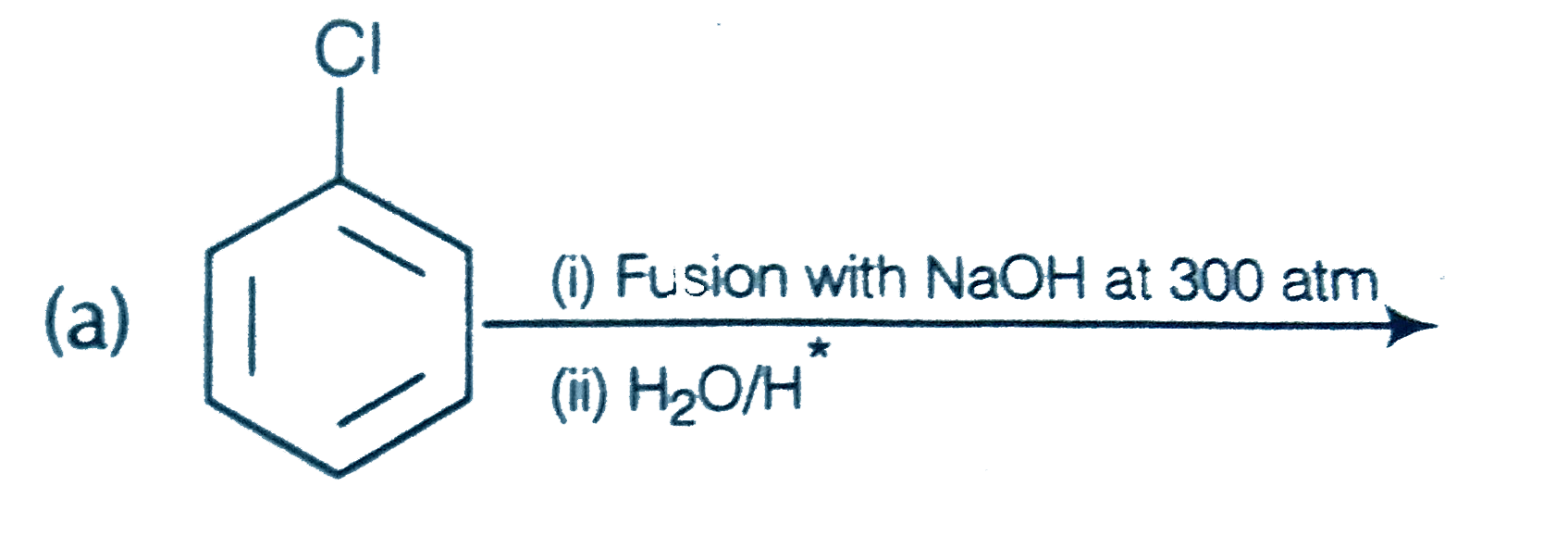
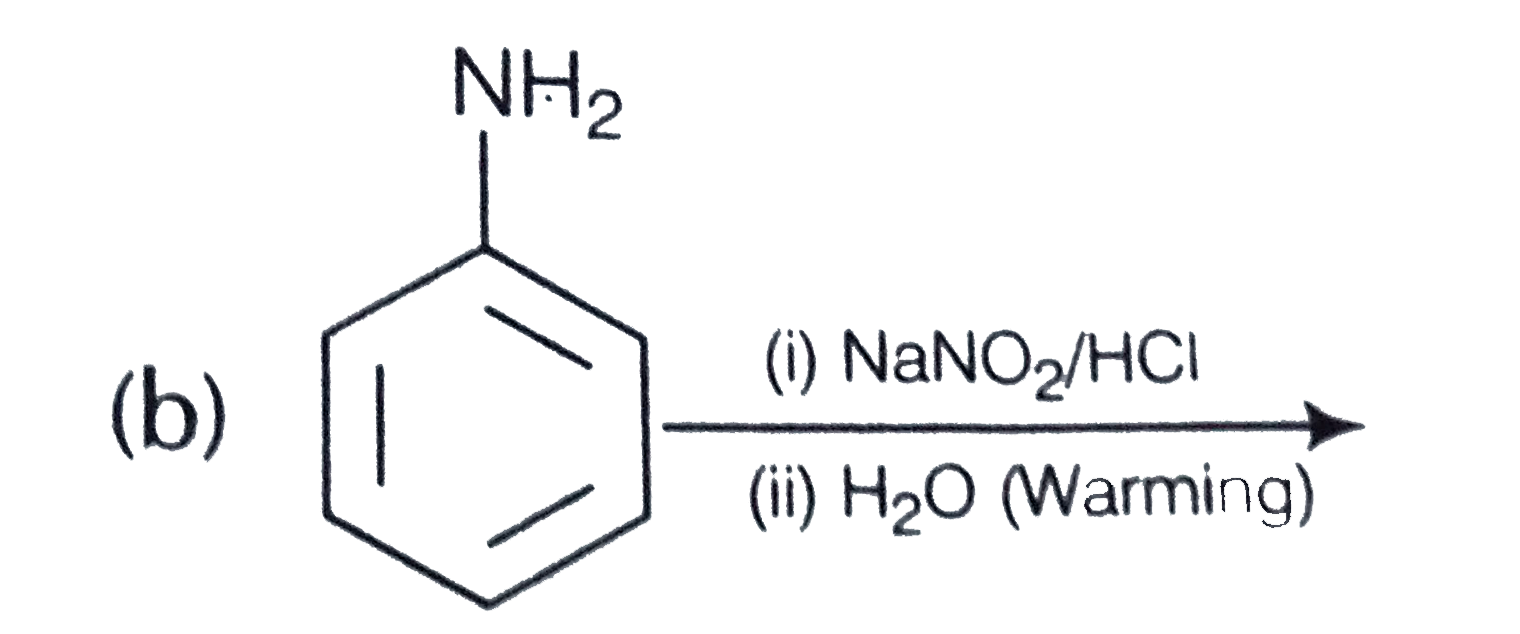
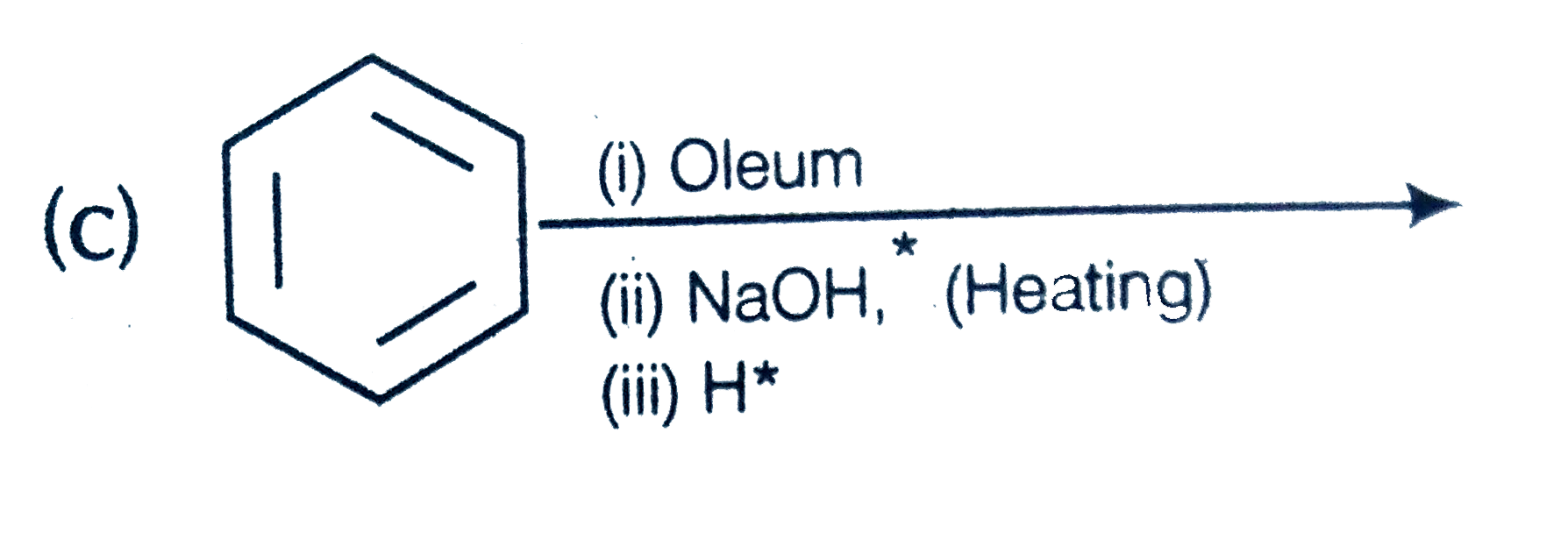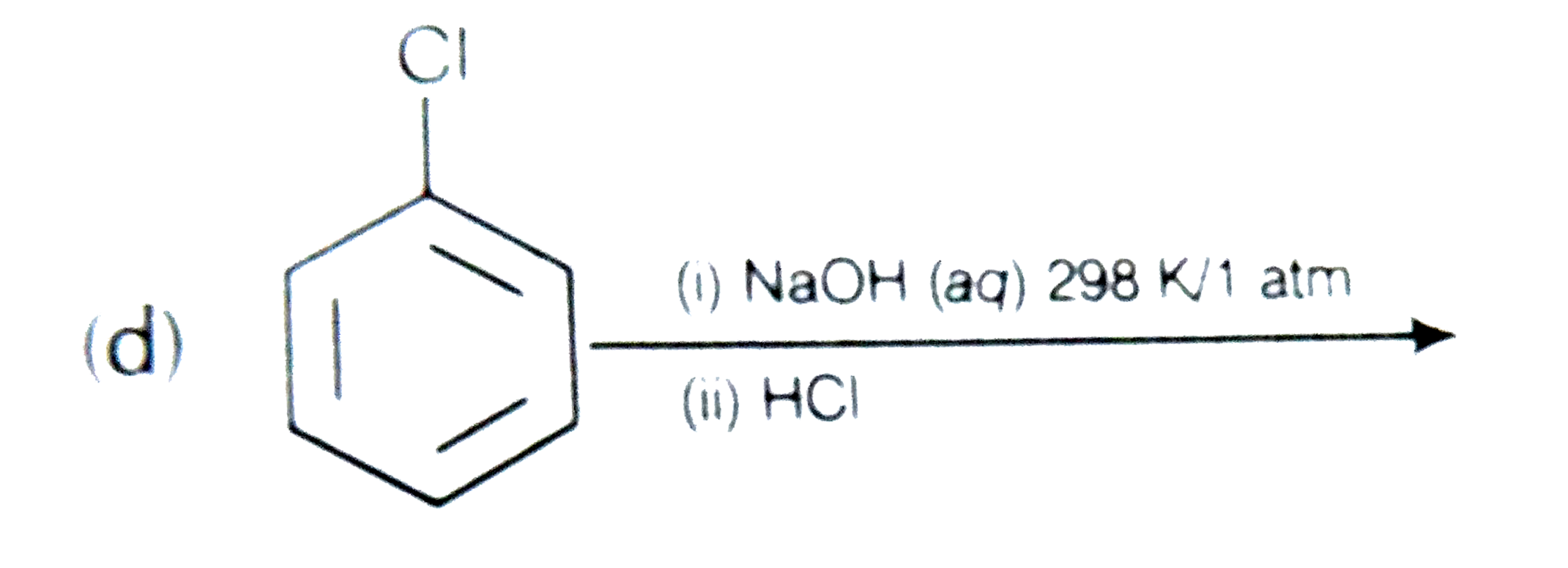A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-ALCOHOLS, PHENOLS AND ETHERS-All Questions
- Arrange the following compounds in increasing order of boiling point :...
Text Solution
|
- Which of the following are used to convert RCHO into RCH(2)OH ?
Text Solution
|
- Which of the following reactions will yield phenol ?
Text Solution
|
- Which of the following reagents can be used to oxidise primary alcohol...
Text Solution
|
- Phenol can be distinguished from ethanol by the following reagents exc...
Text Solution
|
- Which of the following are benzylic alcohols ?
Text Solution
|
- What is the structure and IUPAC name of glycerol ?
Text Solution
|
- Write the IUPAC name of the following compounds. (a) CH(3)-underset(...
Text Solution
|
- Write the IUPAC name of the compound given below. {:(CH(3)-CH(2)-C" ...
Text Solution
|
- Name the factors responsible for the solubility of alcohols in water.
Text Solution
|
- What is denatured alcohol ?
Text Solution
|
- Suggest a reagent for the following conversion.
Text Solution
|
- Out of 2-chloroethanol and ethanol which is more acidic and why ?
Text Solution
|
- Suggest a reagent for conversion of ethanol to ethanal.
Text Solution
|
- Suggest a reagent for conversion of ethanol to ethanoic acid.
Text Solution
|
- Out of o-nitrophenol and p-nitrophenol, which is more volatile ? Expla...
Text Solution
|
- Out of o-nitrophenol and o-cresol which is more acidic ?
Text Solution
|
- When phenol is treated with bromine water, white precipitate is obtain...
Text Solution
|
- What is the correct increasing order of acidic strength in the followi...
Text Solution
|
- Alcohols react with active metals e.g., Na, K etc., to give correspond...
Text Solution
|