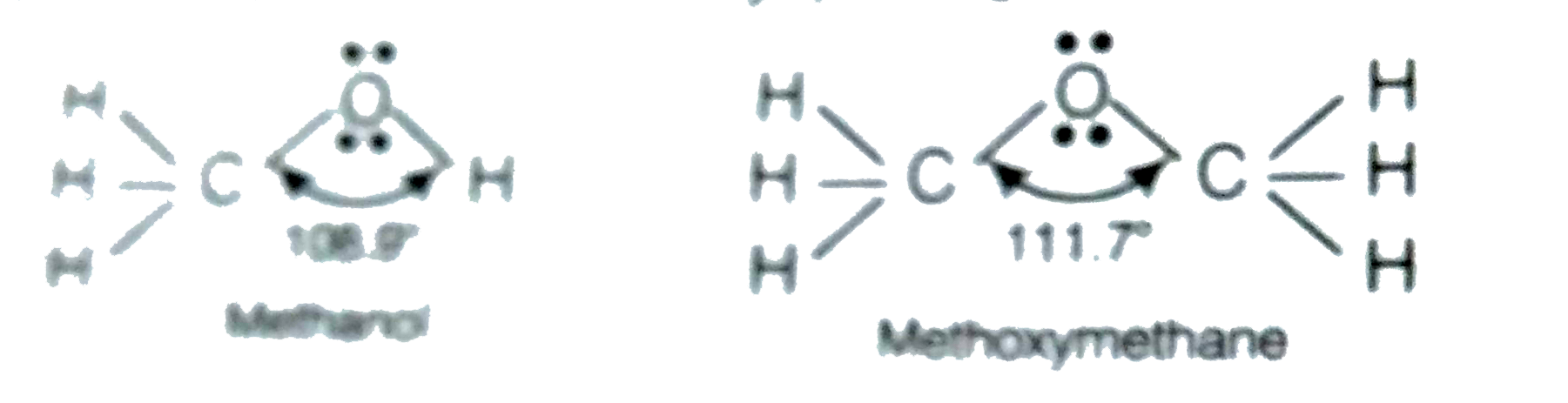Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-ALCOHOLS, PHENOLS AND ETHERS-All Questions
- Dipole moment of phenol is smaller than that of methanol. Why ?
Text Solution
|
- Ethers can be prepared by Williamson synthesis which an alkyl halide i...
Text Solution
|
- Why is the C-O-H bond angle in alcohols slightly less than the tetrahe...
Text Solution
|
- Explain why low molecular mass alcohols are soluble in water ?
Text Solution
|
- Explain why p-nitrophenol is more acidic than phenol ?
Text Solution
|
- Explain why alcohols and ethers of comparable molecular mass have diff...
Text Solution
|
- The carbon-oxygen bond in phenol is slightly stronger than that in met...
Text Solution
|
- Arrange water, ethanol and phenol in increasing order of acidity and g...
Text Solution
|
- Match the structures of the compounds given in Column I with the name ...
Text Solution
|
- Match the starting material given in Column I with the products formed...
Text Solution
|
- Match the items of Column I with items of Column II.
Text Solution
|
- Match the items of Column I with items of Column II.
Text Solution
|
- Assertion (A) Addition reaction of water to but-1-ene in acidic medium...
Text Solution
|
- Assertion (A) p-nitrophenol is more acidic than phenol. Reason (R) N...
Text Solution
|
- Assertion (A) IUPAC name of the compound CH(3)-underset(CH(3))unders...
Text Solution
|
- Assertion (A) Bond angle is ethers is slightly less than tetrahedral a...
Text Solution
|
- Assertion (A) Boiling points of alcohols and ethers are high. Reason...
Text Solution
|
- Assertion (A) Like bromination of benzene, bromination of phenol is al...
Text Solution
|
- Assertion (A) o-nitrophenol is less soluble in water than the m and p-...
Text Solution
|
- Assertion (A) Ethanol is a weaker acid than phenol. Reason (R) Sodiu...
Text Solution
|