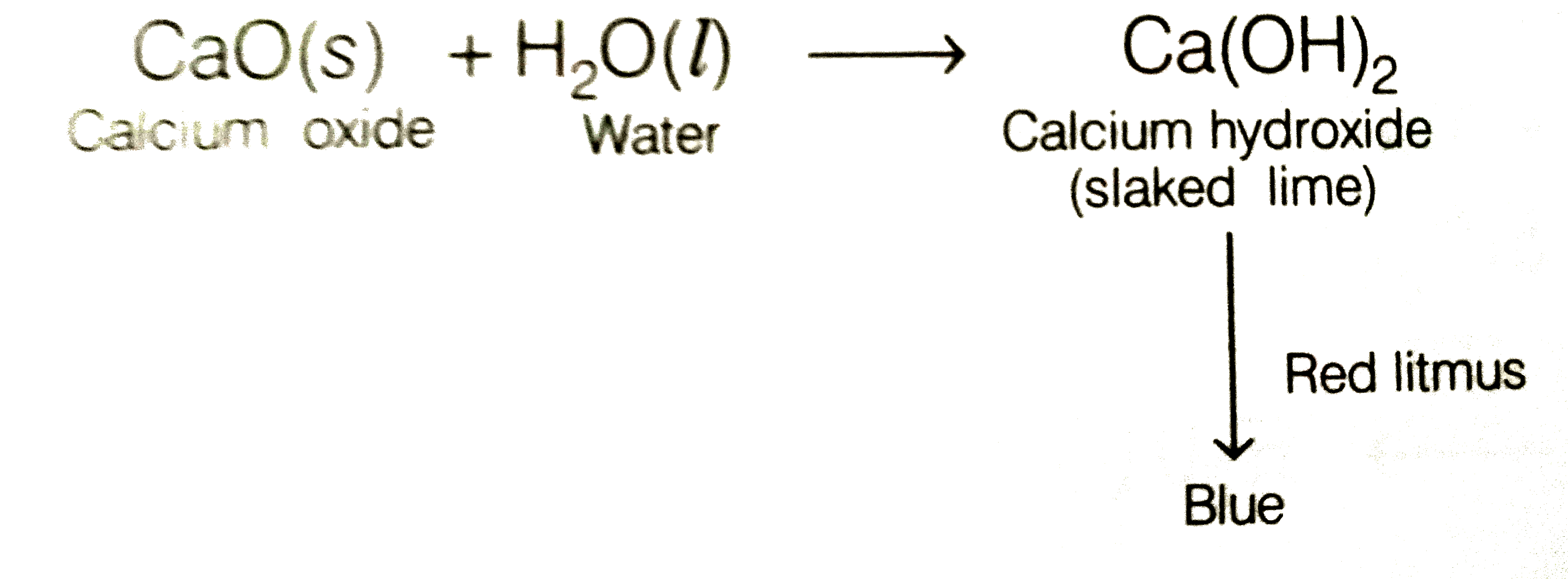
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A substance X, which is an oxide of a group 2 element, is used intensi...
Text Solution
|
- A substance X, which is an oxide of a group 2 element, is used intensi...
Text Solution
|
- An element reacts with oxygen to form an oxide which dissolves in dilu...
Text Solution
|
- A substance X which is an oxide of a metal is used intensively in the ...
Text Solution
|
- An element X forms an oxide which turns red litmus blue. Identify wh...
Text Solution
|
- A substance X, which is an oxide of a group 2 element, is used intensi...
Text Solution
|
- X समूह 2 के एक तत्व का ऑक्साइड है , जो सीमेंट उद्योग में बहुत अधिक उपय...
Text Solution
|
- An element x reacts with oxide to from oxide,X2O which dissolves in wa...
Text Solution
|
- An element combines with oxygen to form an oxide. This oxide dissolves...
Text Solution
|