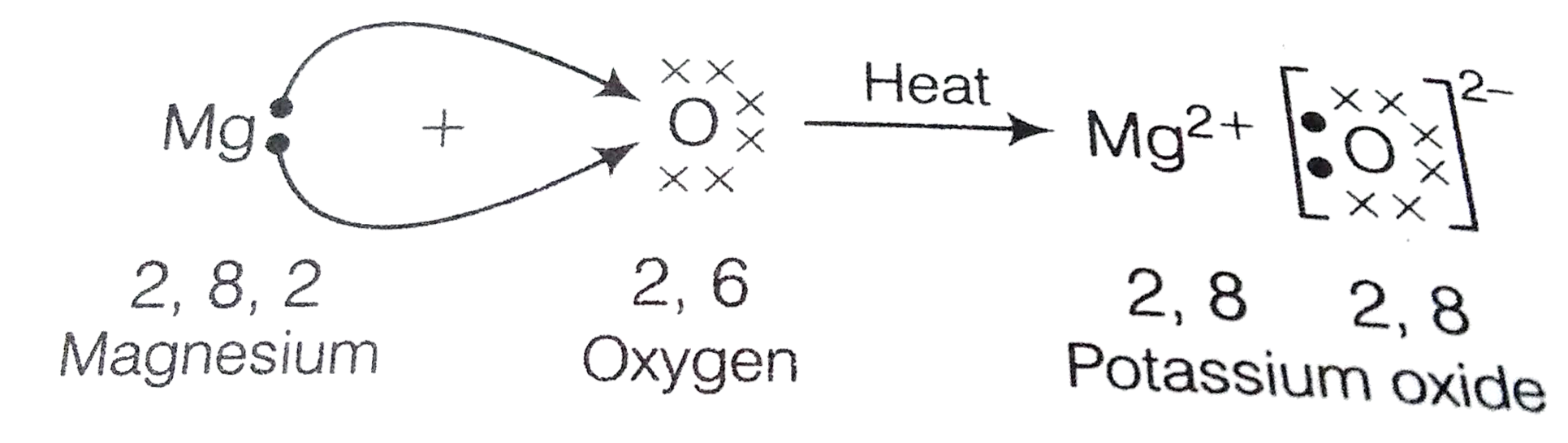
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- An element is placed in 2nd group and 3rd period of the periodic table...
Text Solution
|
- An element is placed in 2nd group and 3rd period of the periodic table...
Text Solution
|
- An element is placed in 2nd group and 3rd period of the period...
Text Solution
|
- An element is placed in 2nd group and 3rd period of the periodic table...
Text Solution
|
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- An element 'X' (Atomic number = 20) burns in the presence of oxygen to...
Text Solution
|
- An element is placed in 2nd Group and 3rd period of Periodic Table, it...
Text Solution
|