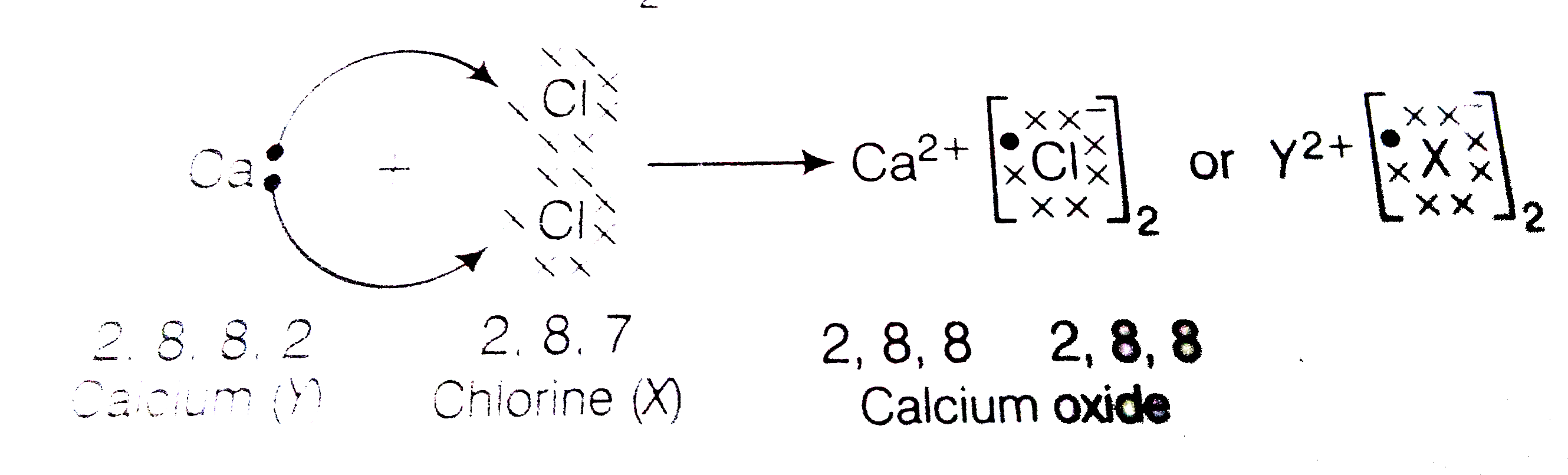Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- दो तत्व X तथा Y जिनके परमाणु क्रमांक क्रमशः 11 तथा 17 है- इन तत्वों ...
Text Solution
|
- The atomic number of an element “X' is 20. (i) Determine the posi...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- The atomic number of an element 'X' is 20. (i) Determine the positio...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- An element 'X' with atomic number 11 forms a compound with element 'Y'...
Text Solution
|
- An element X with atomic number 12 forms a compound with element Y wit...
Text Solution
|