Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
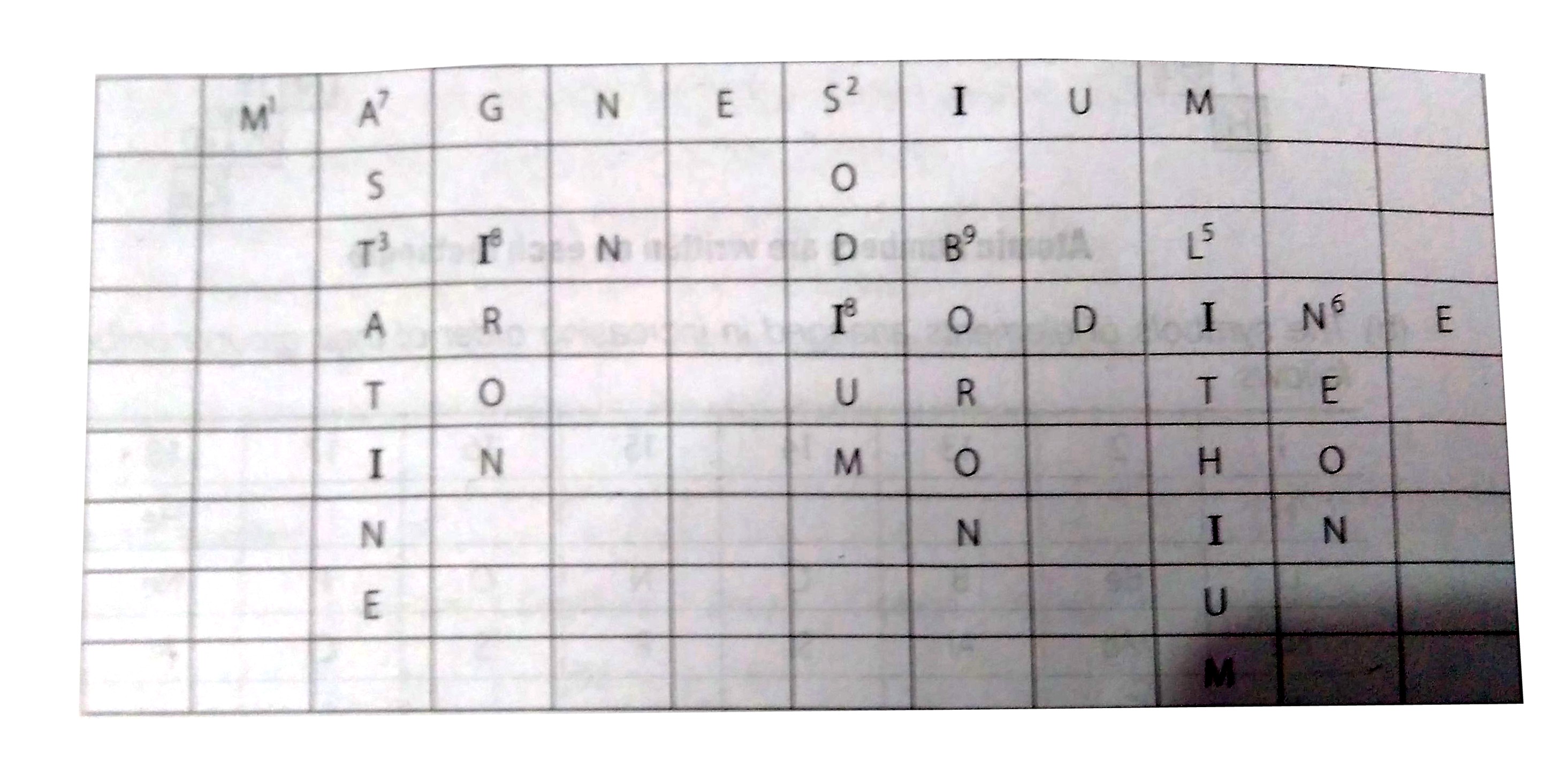
- Complete the following crossword puzzle (Figure) Across (1) An ele...
Text Solution
|
- Complete the following crossword puzzle (Figure) Across (1) An element...
Text Solution
|
- An atom of an elements has two electrons in outermost M shell state it...
Text Solution
|
- Name the following : (a) A metal which is preserved in kerosene (b) A ...
Text Solution
|
- The atomic number of.an elements is 16. Predict 1. The number of valen...
Text Solution
|
- Name three elements with seven valence electrons in their outermost sh...
Text Solution
|
- Name the following : (i) Metal, which is preserved in kerosene. (ii) A...
Text Solution
|
- Complete the following crossword puzzle (Figure) Across (1) An ele...
Text Solution
|
- Complete the following cross-word puzzle : Across : (1) An elem...
Text Solution
|