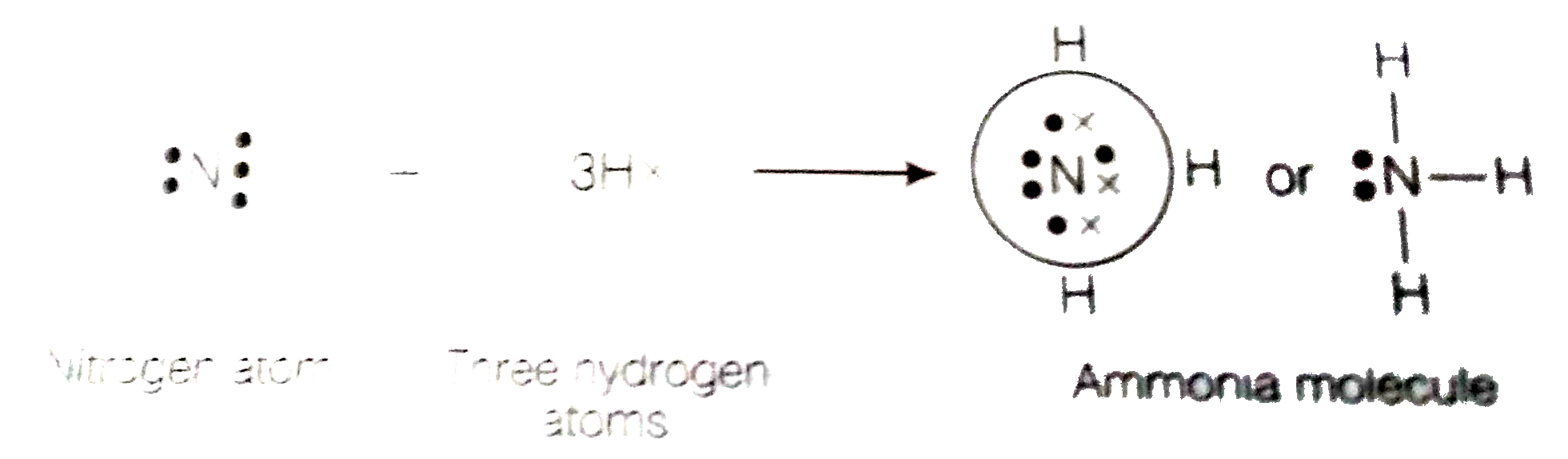Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
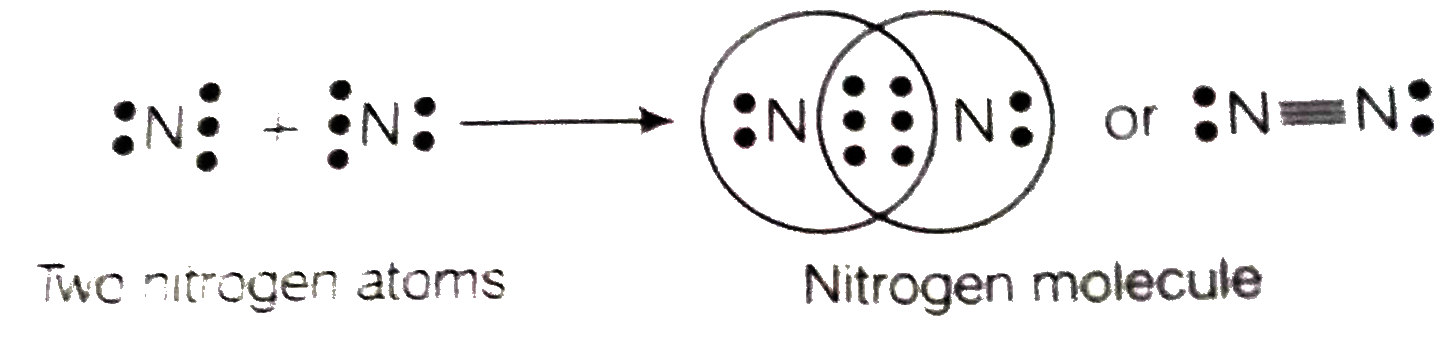
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- An element forms diatomic molecule with a triple bond. The configurati...
Text Solution
|
- एक तत्व A के संयोजी शॉल में 4 इलेक्ट्रॉन हैं और दूसरे तत्व B के संयोजी...
Text Solution
|
- Assertion : In atomic form hydrogen consists of one proton and one ele...
Text Solution
|
- How is the compound formed when the element A having electronic config...
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- Which element does not form stable diatomic molecule ?
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|