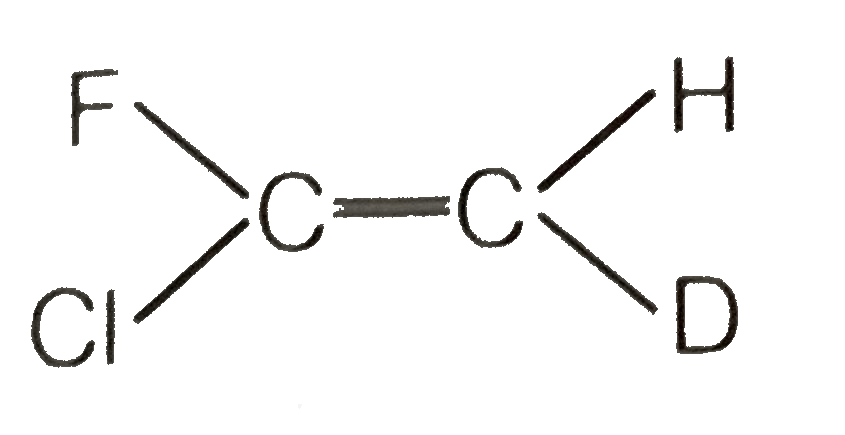
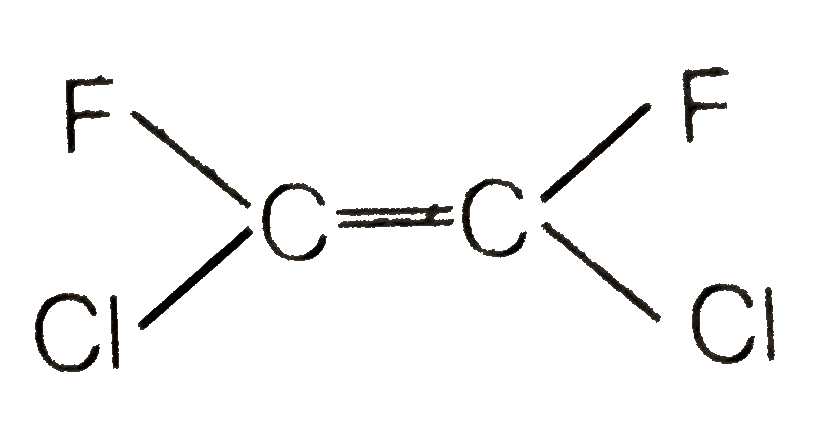
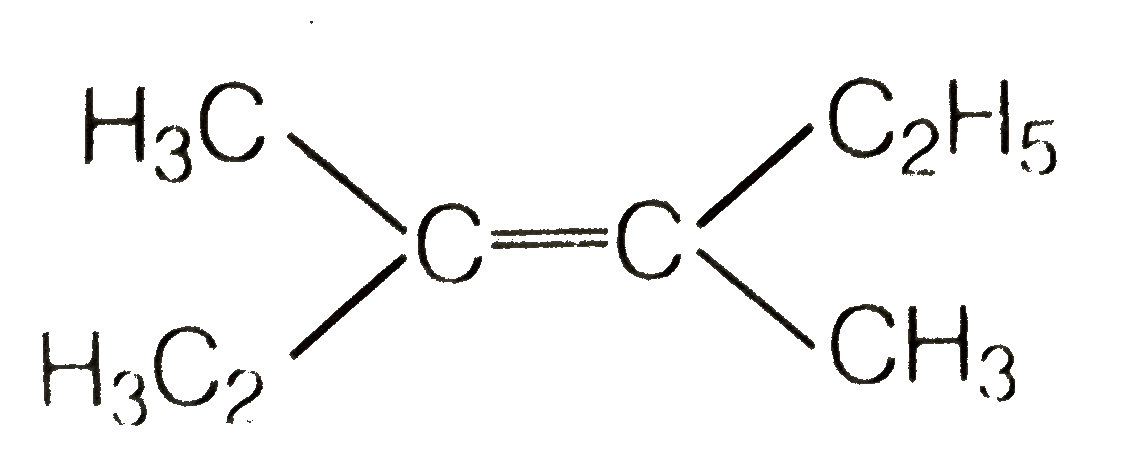
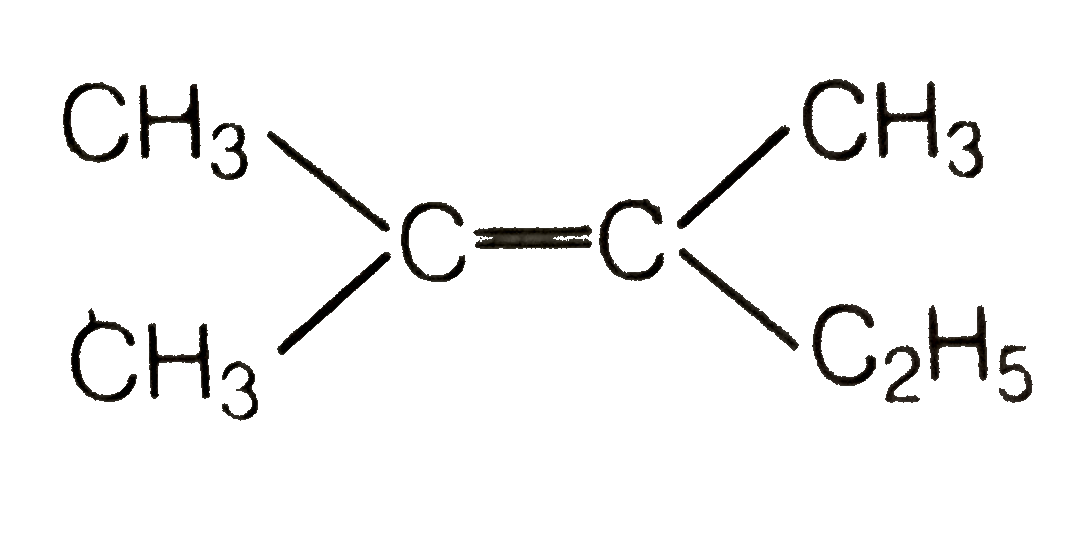
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NCERT EXEMPLAR ENGLISH|Exercise Multiple Choice Questions (More Than One Options)|9 VideosHYDROCARBONS
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Questions|20 VideosENVIRONMENTAL CHEMISTRY
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type|5 VideosHYDROGEN
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|8 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-HYDROCARBONS-Long Answer Type Questions
- Which of the following will not show geometrical isomerism ?
Text Solution
|
- An alkyl halide C(5)H(11) (A) reacts with ethanolic KOH to give an alk...
Text Solution
|
- 896 mL vapour of a hydrocarbon 'A' having carbon 87.80% and hydrogen 1...
Text Solution
|
- An unsaturated hydrocarbon 'A' adds two molecules of H(2) and on reduc...
Text Solution
|
- In the presence of peroxide addition of HBr to propene takes place acc...
Text Solution
|