Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR ENGLISH|Exercise Short answer types questions|19 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR ENGLISH|Exercise Matching the Columns|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR ENGLISH|Exercise Long Answer type questions|9 VideosENVIRONMENTAL CHEMISTRY
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type|5 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.-Long answer types question
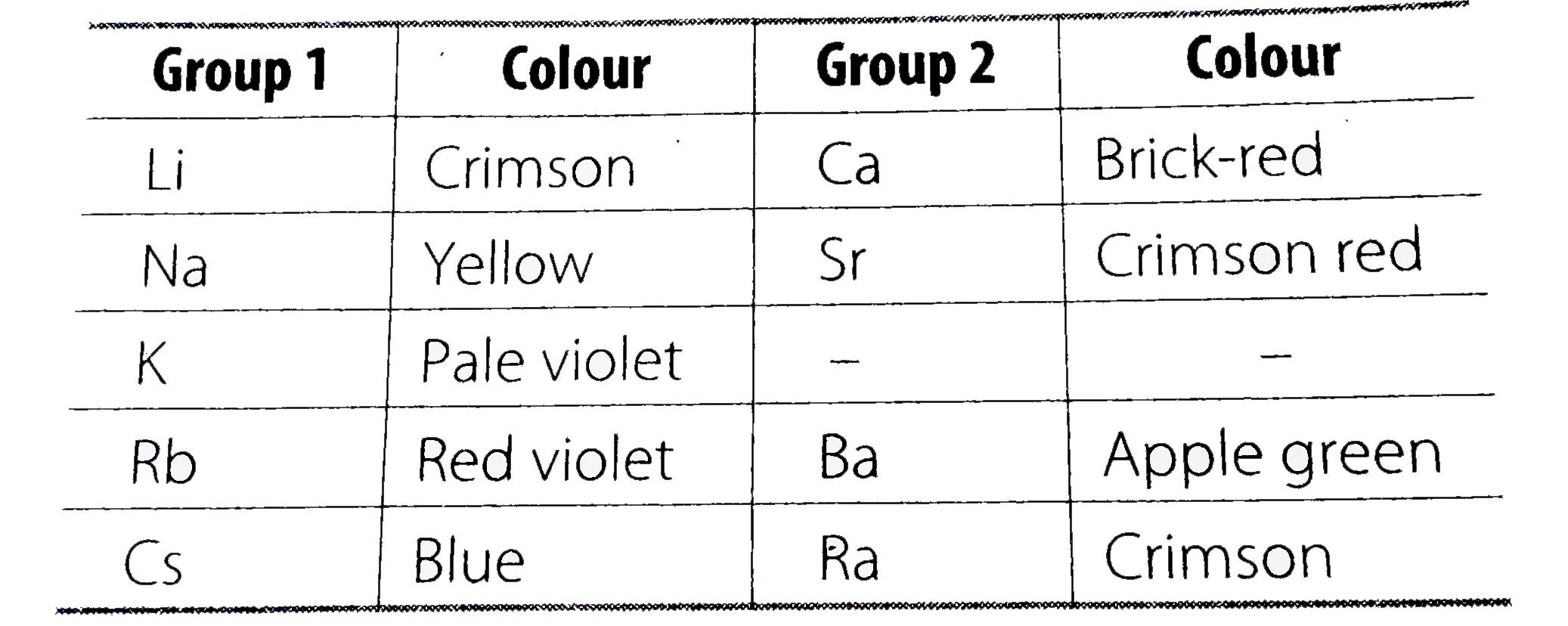
- Those elements impart colour to hte flame on heating in it, the atoms ...
Text Solution
|
- Discuss the factors affecting electron gain enthalpy and the trend in ...
Text Solution
|
- Define ionisation enthalpy. Discuss the factors affecting ionisation e...
Text Solution
|
- Justify the given statement with suitable examples -"the properties of...
Text Solution
|
- Write down the outermost electronc configuration of alkali metals. How...
Text Solution
|
- Write the drawbacks in Mendeleev's periodic table that let to its modi...
Text Solution
|
- In what manner is the long form of periodic table better than Mendele...
Text Solution
|
- Discuss and compare the trend in ionisation enthalpy of the elements o...
Text Solution
|