Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Match the Column|6 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Assertion and Reason|14 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Qns|4 VideosCHEMICAL KINETICS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|5 VideosCOORDINATION COMPOUNDS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Question|5 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-CHEMISTRY IN EVERYDAY LIFE-Short Answer Type
- Dishwashing soaps are synthetic detergents. What is their chemical nat...
Text Solution
|
- Draw the diagram showing micelle formation by the following detergent....
Text Solution
|
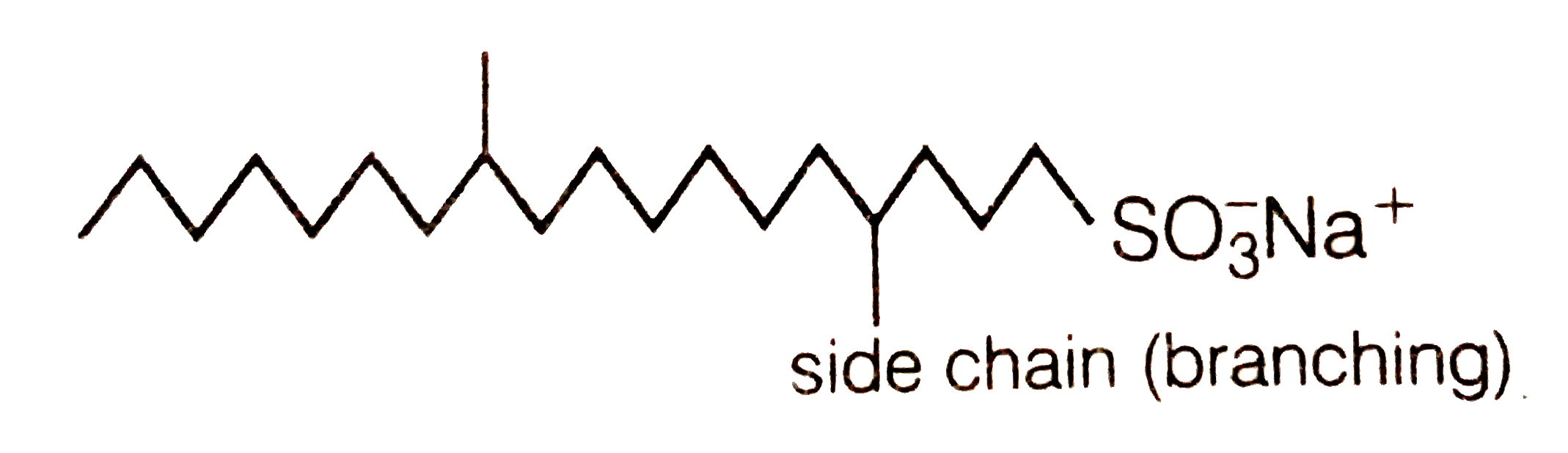
- How does the branching of hydrocarbon chain of synthetic detergents af...
Text Solution
|
- Why is it safer to use soap from the enviornmental point of view ?
Text Solution
|
- What are analgesics ?
Text Solution
|
- What is the scientefic explanation for the feeling depression ?
Text Solution
|
- What is the basic difference between antiseptics ad disinfectants ?
Text Solution
|
- Between sodiumhydrogencarbonate and magnesium hydroxide which is a bet...
Text Solution
|
- Which analgesics are called opiates ?
Text Solution
|
- What is the medicinal use of narcotic drugs ?
Text Solution
|
- What are antagonistric drug ?
Text Solution
|
- What is the mode of action of antimicrobial drugs?
Text Solution
|
- What is the side product of soap industry ? Give reactions showing so...
Text Solution
|
- What is the differene between bathing soap and washing soaps ?
Text Solution
|
- How are transparent soaps manufactured ?
Text Solution
|
- What is the advantage of using antihistamines over antacids in the tre...
Text Solution
|
- What are the functions performed by histamine in the body ?
Text Solution
|
- With the help of an example explain how do transquilizers control the...
Text Solution
|
- Why are certain drugs called enzyme inhibitors ?
Text Solution
|
- What are fillers and what role these fillers play in soap ?
Text Solution
|