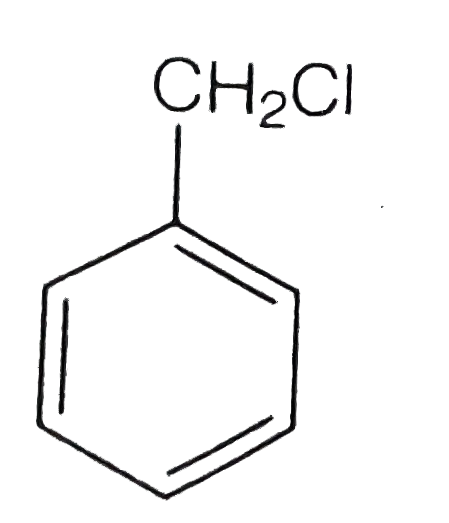
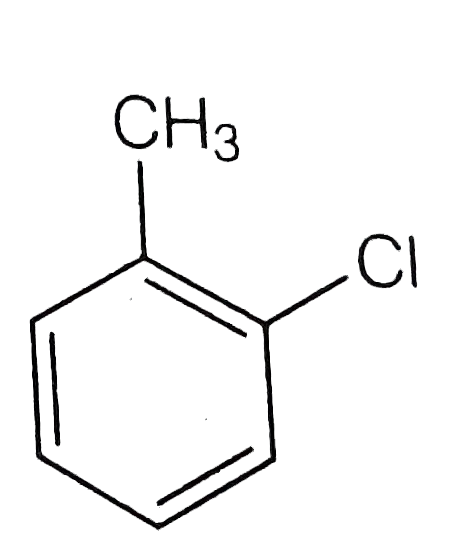
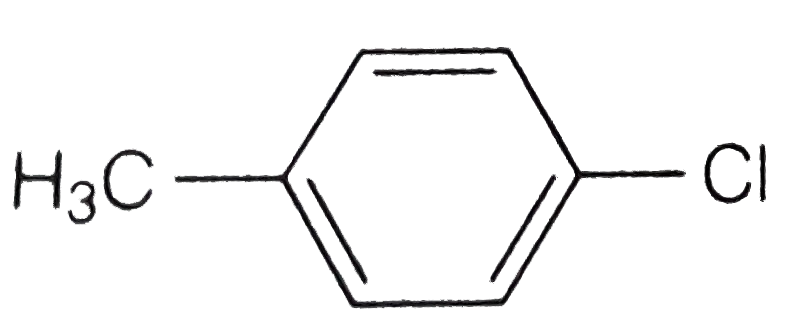
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise MCQs (More than one option)|12 VideosHALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Questions|35 VideosGENERAL PRINCIPLE AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|1 VideosP-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|3 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-HALOALKANES AND HALOARENES-Long Answer Type Questions
- The reaction of toluene with chlorine in the presence of iron and in t...
Text Solution
|
- Some alkyl halides undergo substitution whereas some undergo eliminati...
Text Solution
|
- Some halogen containing compounds are useful in daily life. Some compo...
Text Solution
|
- Why are aryl halides less reactive towards nucleophilic substitution ...
Text Solution
|