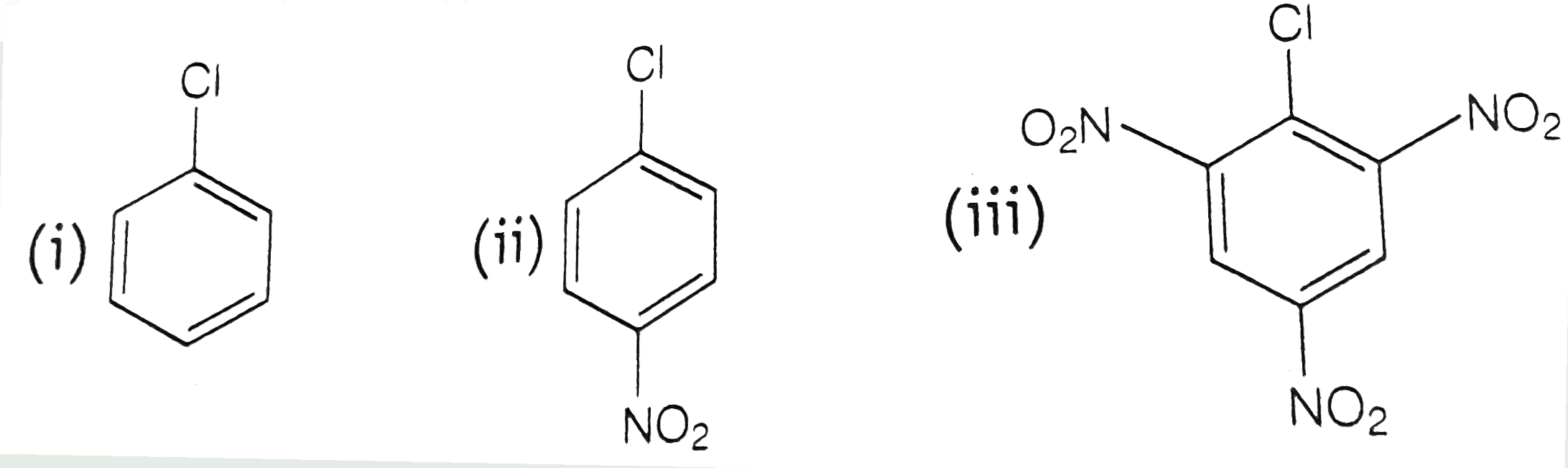
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise MCQs (More than one option)|12 VideosHALOALKANES AND HALOARENES
NCERT EXEMPLAR ENGLISH|Exercise Short Answer Type Questions|35 VideosGENERAL PRINCIPLE AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|1 VideosP-BLOCK ELEMENTS
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|3 Videos