A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT EXEMPLAR ENGLISH|Exercise MULTIPLE CHOICE QUESTIONS (MORE THAN ONE OPTIONS|28 VideosSOLUTIONS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|8 VideosSOLID STATE
NCERT EXEMPLAR ENGLISH|Exercise long answer type questions|4 VideosSURFACE CHEMISTRY
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-SOLUTIONS -LONG ANSWER TYPE QUESTIONS
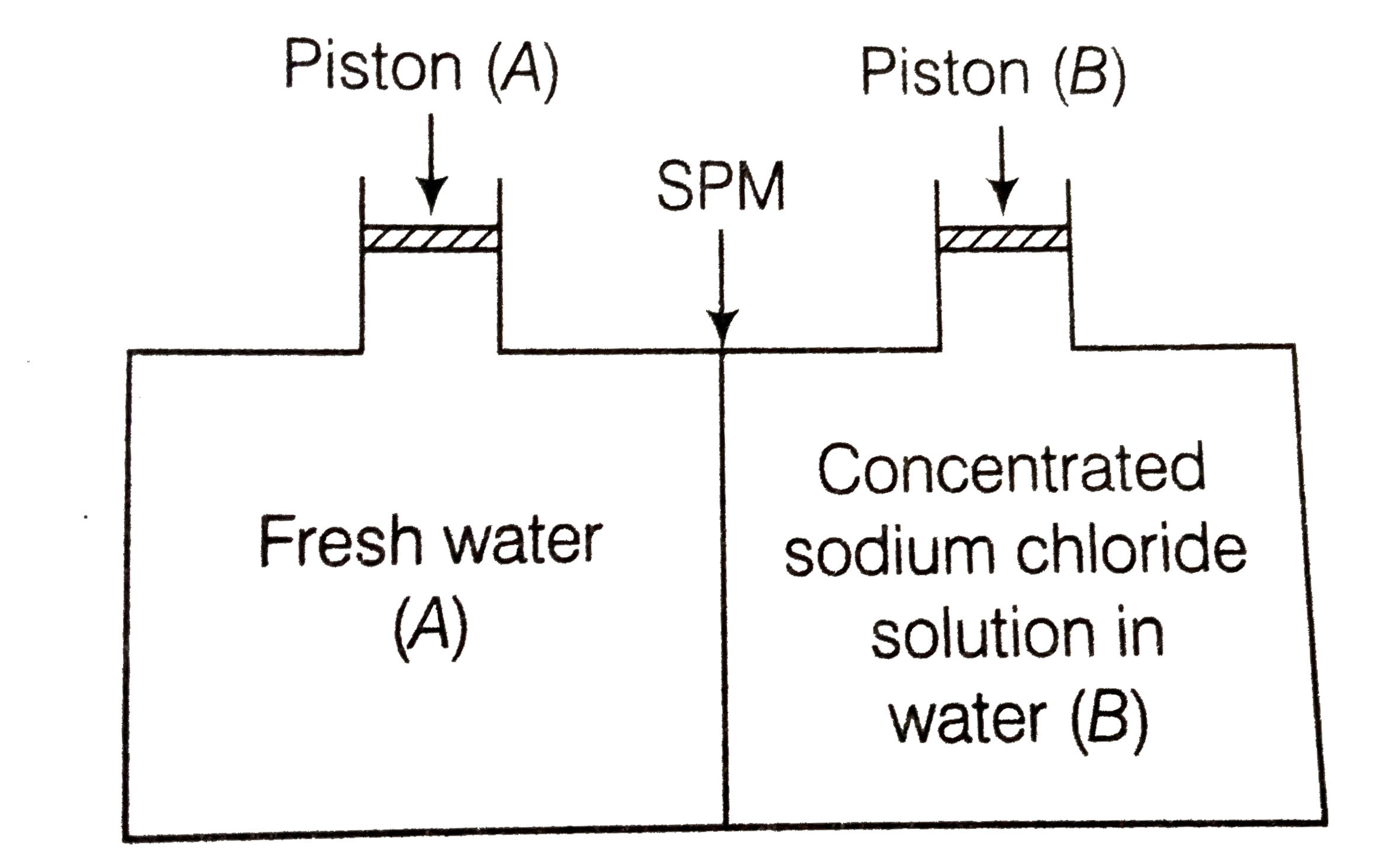
- Consider the figure and mark the correct option.
Text Solution
|
- Diffine the following mofes of expressing the concentration of a solut...
Text Solution
|
- Using Raoult's Law explain how the total vapour pressure over the solu...
Text Solution
|
- Explain the terms ideal and non-idealsolution in the light of forces o...
Text Solution
|
- Why is it not possible to obtain pure ethanol by fractional distillati...
Text Solution
|
- When kept in water, raisin swells in size. Name and explain the phenom...
Text Solution
|
- Discuss biological and industrial applications of osmosis.
Text Solution
|
- How can you remove the hard calcium carbonate layer of the egg without...
Text Solution
|
- Why is the mass determined by measuring a colligative property in case...
Text Solution
|