A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
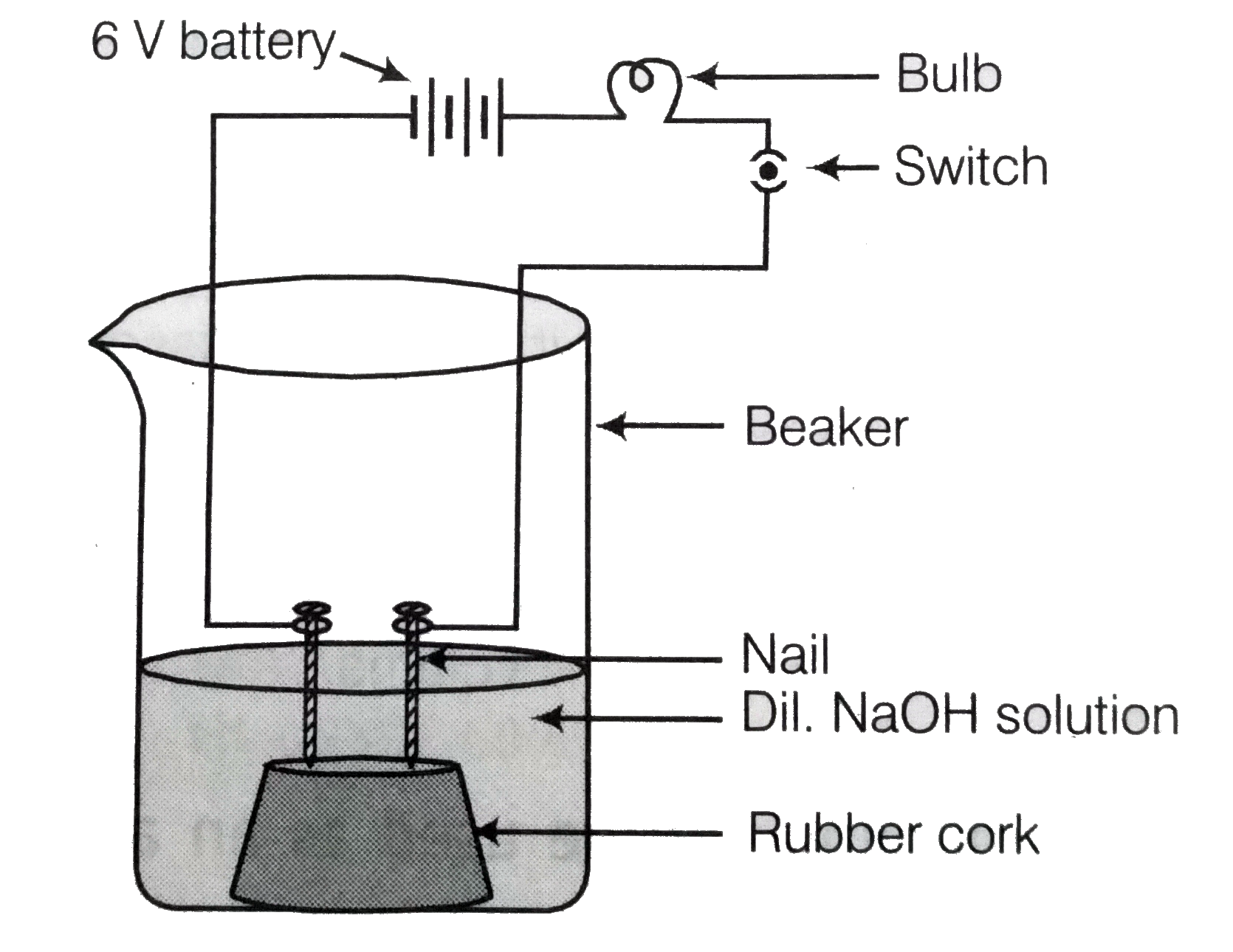
- In an attempt to demonstrate electrical conductivity through an electr...
Text Solution
|
- In an attempt to demonstrate electrical conductivity through an electr...
Text Solution
|
- In an attempt to demonstrate electrical conductivity through an electr...
Text Solution
|
- क्या चित्र में दिखाए गए परिपथ में बल्ब दीप्तिमान होगा?
Text Solution
|
- In an attempt to demonstrate electrical conductivity through an electr...
Text Solution
|
- In which among the following circutis does the bulb glow?
Text Solution
|
- क्या चित्र में दिखाए गए परिपथ में बल्ब दीप्तिमान होगा?
Text Solution
|
- Which energy is cause for glowing of bulb in electrolytic cell?
Text Solution
|
- In the following figures, the bulbs in Figure A and D glow, but the bu...
Text Solution
|