Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
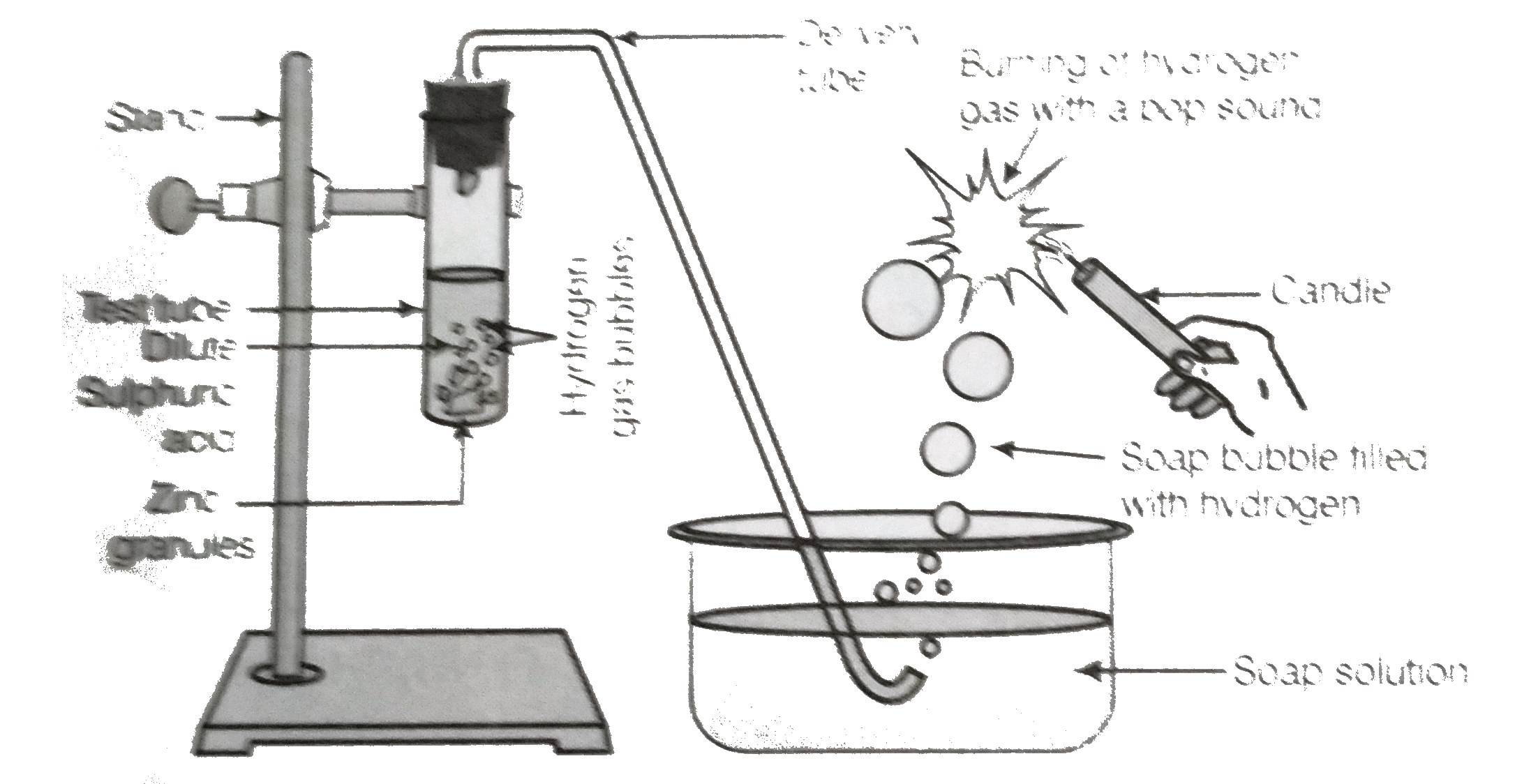
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- When dilute sulphuric acid is added to granulated zinc placed in a tes...
Text Solution
|
- When dilute hydrochloric acid is added to granulated zinc placed in a ...
Text Solution
|
- In the schematic diagram for the preparation of hydrogen gas as shown ...
Text Solution
|
- (i) In the following schematic diagram for the preparation of hydrogen...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- When dilute hydrochloric acid is added to zinc pieces taken in a test ...
Text Solution
|