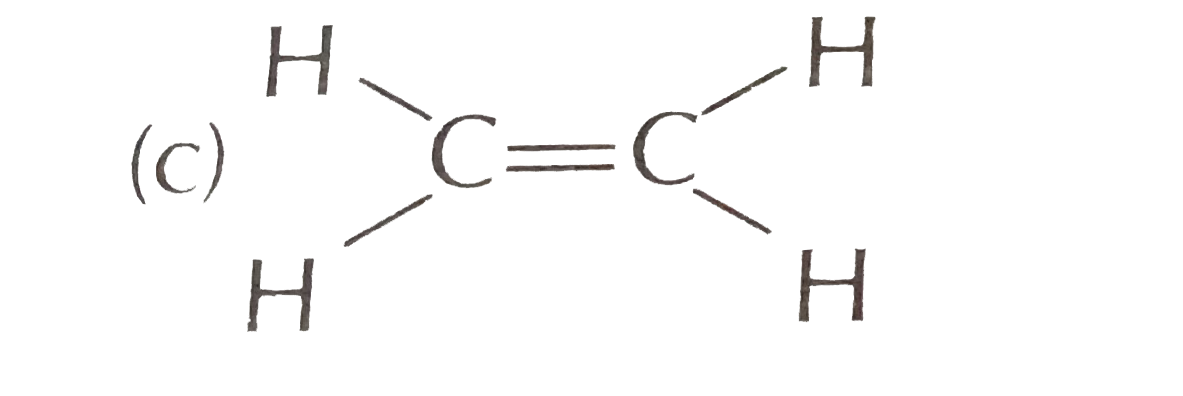
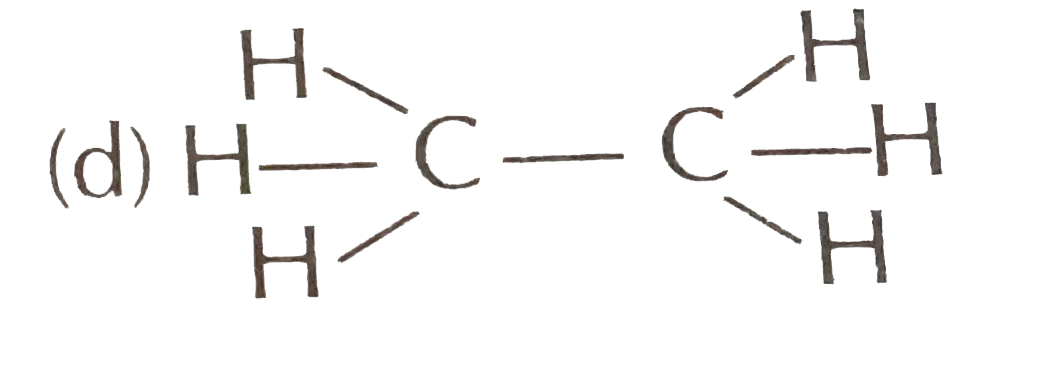
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Structural formula of ethyne is
Text Solution
|
- Structural formula of ethyne is
Text Solution
|
- Draw the electron dot structure of ethyne and also draw its structure ...
Text Solution
|
- Draw the electron- dot structure and structural formula of ethyne.
Text Solution
|
- The molecular formula of ethyne is C(2)H(2). From this, draw its struc...
Text Solution
|
- Draw the electron dot structure of ethyne and also draw its structural...
Text Solution
|
- Write the molecular, electron-dot , and structural formula of ethyne.
Text Solution
|
- Structural formula of ethyne is
Text Solution
|
- Draw the electron dot structure of ethyne and also draw its structure ...
Text Solution
|