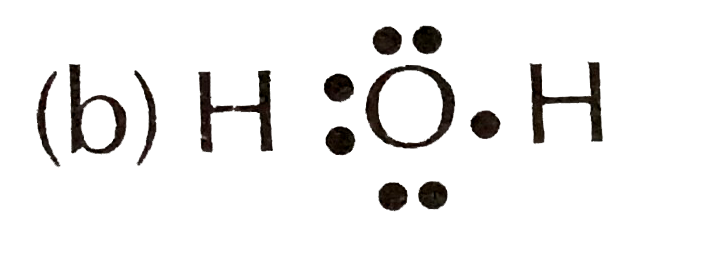A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct electron dot structure of a water molecule is
Text Solution
|
- Which of the following is the correct electron-dot structure of N2O mo...
Text Solution
|
- The correct electron dot structure of a water molecule is
Text Solution
|
- Which is the correct electron dot structure of N(2)O molecules
Text Solution
|
- Draw electron-dot and line structure of an ethane molecule.
Text Solution
|
- Draw the electron-dot structure of the molecules (without showing cir...
Text Solution
|
- Draw an electron dot structure of the molecules. (without showing the ...
Text Solution
|
- Which of the following is the correct electron dot structure of N(2)O ...
Text Solution
|
- The correct electron dot structure of a water molecule is
Text Solution
|