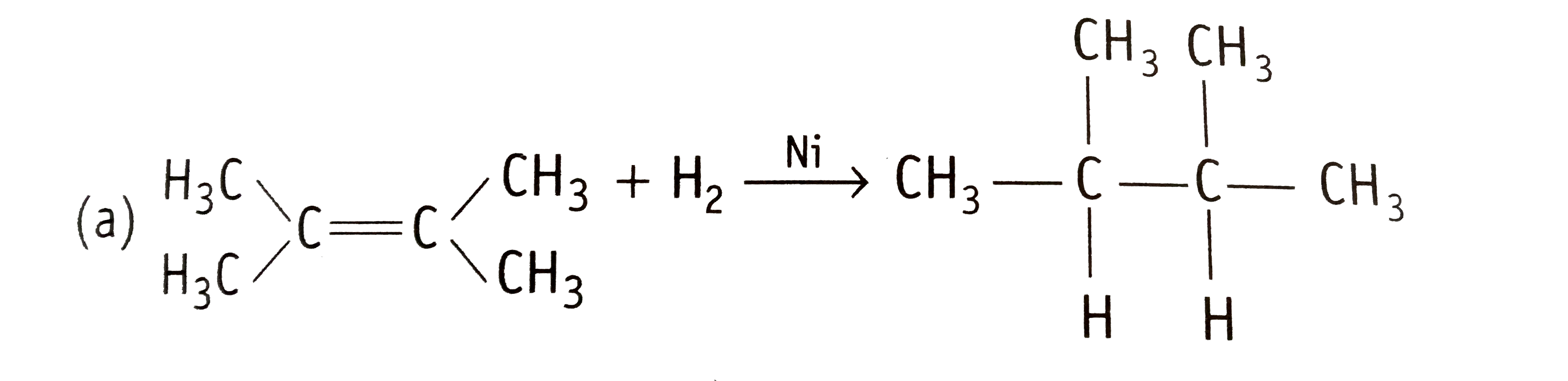Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the role of metal or reagents written on arrows in the given c...
Text Solution
|
- CH(3)-CH(2)-OHoverset("Alkaline"KMnO(4)+"Heat")(to)CH(3)-COOH In the...
Text Solution
|
- What is the role of metal or reagents written on arrows in the given c...
Text Solution
|
- Write mechanism of the following reaction: CH(3)CH(2)Ohoverset(HBr)toC...
Text Solution
|
- State the role of reagents shown on arrows in the following chemical r...
Text Solution
|
- State the role of reagents shown on arrows in the following chemical r...
Text Solution
|
- State the role of reagents shown on arrows in the following chemical r...
Text Solution
|
- The given reaction is CH(3)COOH + C(2)H(5)OH overset("Conc." H(2)SO(4)...
Text Solution
|
- What is the role of metal or reagents written on arrows in the given c...
Text Solution
|