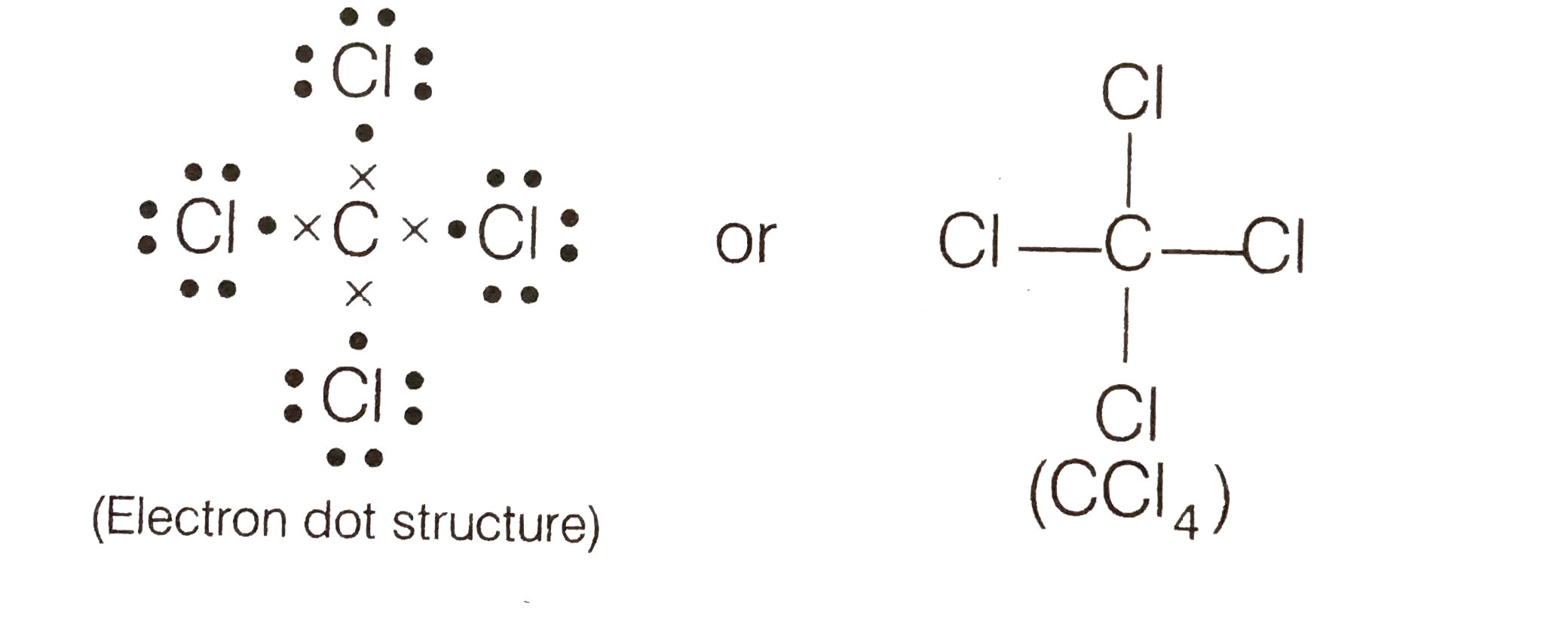Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Write the formula and draw electron dot structure of carbon tetra...
Text Solution
|
- (a) Write the formula and draw electron dot structure of carbon tetrac...
Text Solution
|
- Draw the electron- dot structure and structural formula of ethyne.
Text Solution
|
- ಕಾರ್ಬನ್ ಡೈಆಕ್ಸೈಡ್ ನ ಅಣುಸೂತ್ರ CO2 , ಇದರ ಇಲೆಕ್ಟ್ರಾನ್ ಚುಕ್ಕಿ ರಚನೆಯನ್ನು ಬರ...
Text Solution
|
- Draw the electron dot structure of ethyne and also draw its structural...
Text Solution
|
- কার্বনের যোজ্যতা 4 ও ক্লোরিনের যোজ্যতা 1হলে কার্বন টেট্রাক্লোরাইডের সং...
Text Solution
|
- Write the molecular, electron-dot , and structural formula of ethyne.
Text Solution
|
- Write the molecular formula and electron dot structure of a] Ethane...
Text Solution
|
- (a) Write the formula and draw electron dot structure of carbon tetra...
Text Solution
|