Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Esters are sweet-smelling substances and are used in making perfumes. ...
Text Solution
|
- Esters are sweet-smelling substances and are used in making perfumes. ...
Text Solution
|
- (a) Esters are sweet smelling substances and are used in making perfum...
Text Solution
|
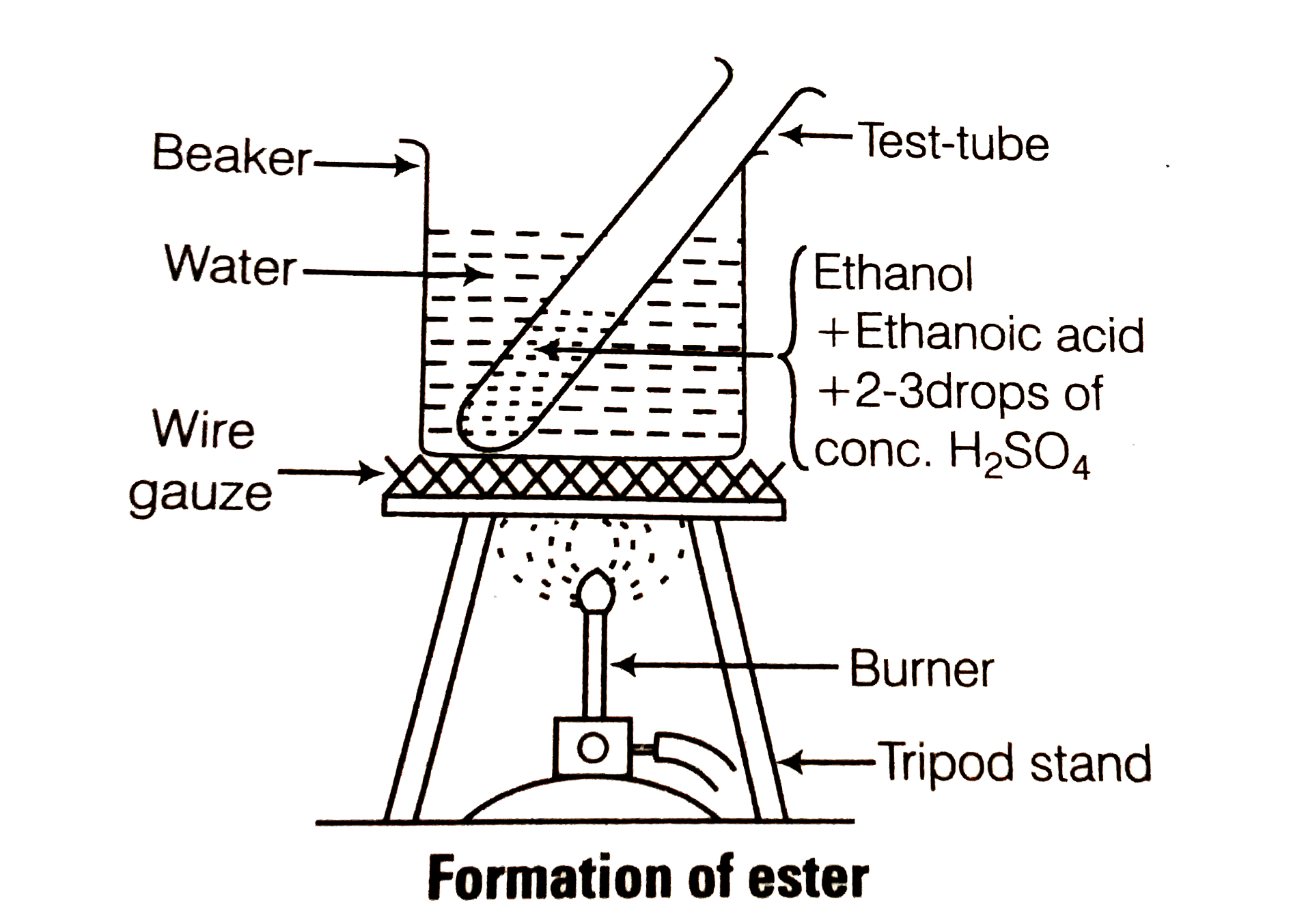
- Describe and activity to show that ehanol and ethanoic acid react to f...
Text Solution
|
- एक एस्टर का नाम बताएँ । एस्टर की गंध कैसी होती है ?
Text Solution
|
- What are esters? Explain the preparation of ethyl ethanoate with the h...
Text Solution
|
- What are esters? Explain the preparation of ethyl ethanoate with the h...
Text Solution
|
- Ester formation involves the reaction of
Text Solution
|
- Ester formation involves the reaction of
Text Solution
|