Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
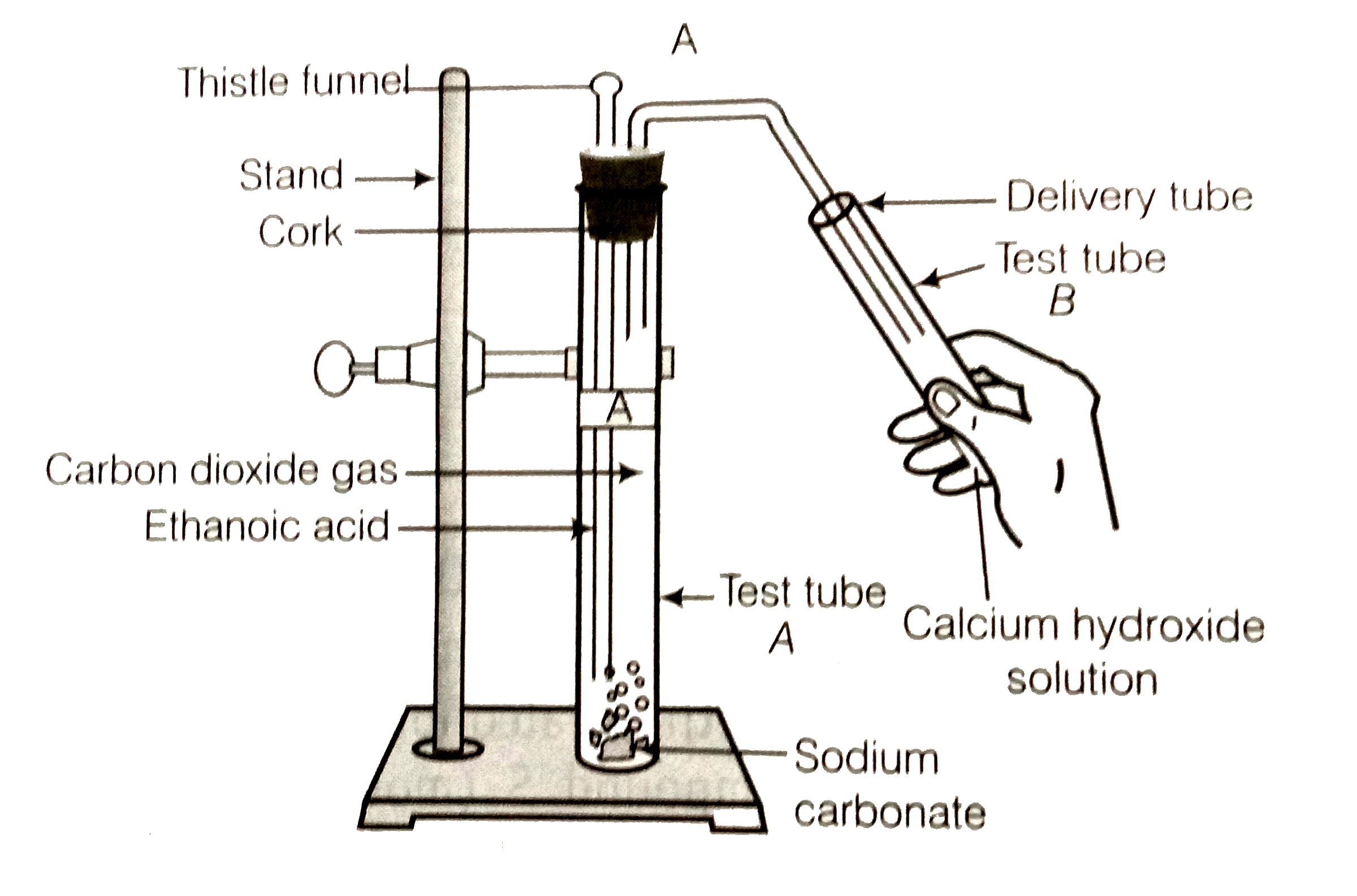
- Look at the figure and answer the following questions. (a) What...
Text Solution
|
- Look at the figure and answer the following questions. (a) What...
Text Solution
|
- Four test tubes were taken and marked A, B, C and D respectively. 2 mL...
Text Solution
|
- Look at the figure and answer the following questions: (a) What change...
Text Solution
|
- (a) You have three unlabelled test tubes containing ethanol, ethanoic ...
Text Solution
|
- Answer question numbers (a) - (d) on the basis of your understanding o...
Text Solution
|
- Look at the figure and answer the following questions. (a) What change...
Text Solution
|
- आप निम्नलिखित परिवर्तन किस प्रकार करेंगे ? प्रक्रिया का नाम दीजिए तथा ...
Text Solution
|
- In the given picture there is a lime water in A and B test tubes. A bo...
Text Solution
|