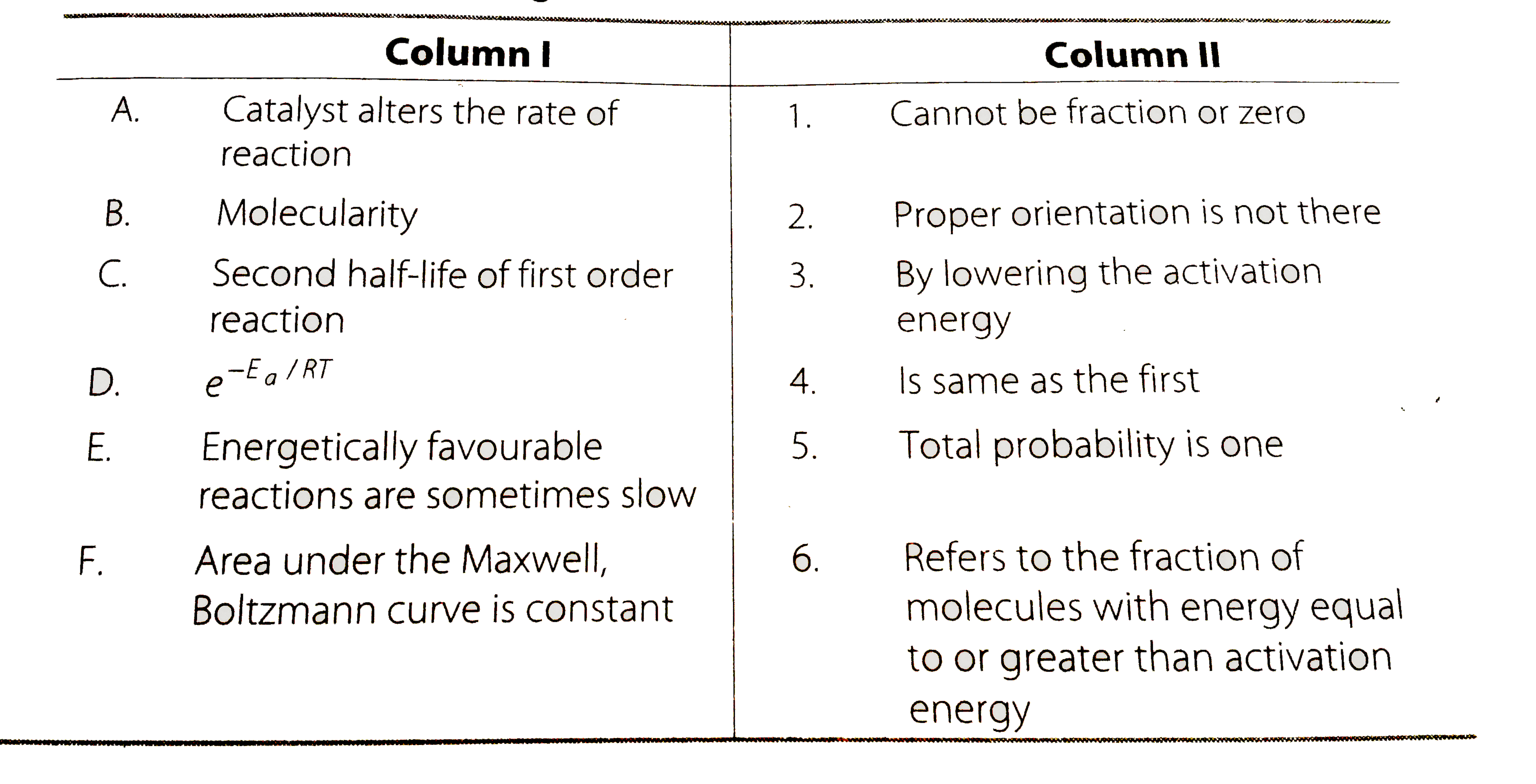
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|5 VideosCHEMICAL KINETICS
NCERT EXEMPLAR ENGLISH|Exercise SHORT ANSWER TYPE QUESTION|20 VideosBIOMOLECULES
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|5 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Qns|4 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-CHEMICAL KINETICS-MATCHING THE COLUMNS
- Match the grap given in column I with the order of reacting given i...
Text Solution
|
- Match the statements given in Column I abd Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Assertion (A) Order of the reaction can be zero or fractional. Reaso...
Text Solution
|
- Assertion (A) Order and molecularity are same. Reason (R) Order is d...
Text Solution
|
- Assertion (A) The enthalpy of reaction remains constant in the presenc...
Text Solution
|
- Assertion (A) All collision of reactant molecules lead to product form...
Text Solution
|
- Assertion (A) Rate constant determined form Arrhenius equations are...
Text Solution
|