Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET-III|31 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise CHEMISTRY (THEORY)|31 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET - II|12 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-SET-II
- Give one example of a condensation polymer.
Text Solution
|
- (a) Why does presence of excess of lithium makes Li Cl crystals pink? ...
Text Solution
|
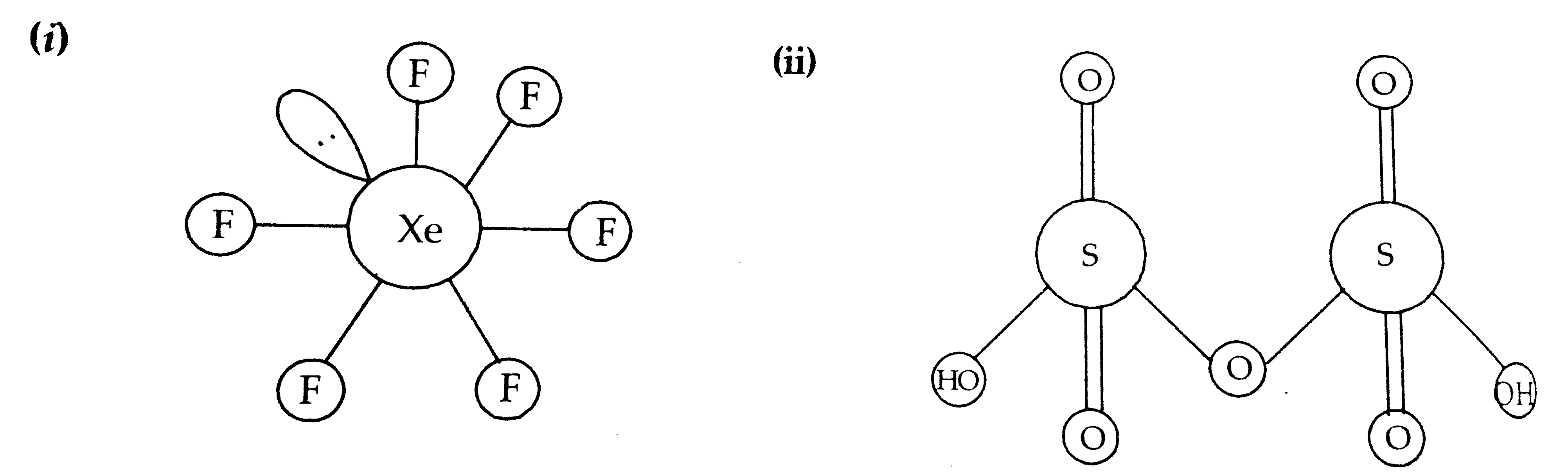
- Draw the structures of the following molecules : (i) XeF(6) (ii) H...
Text Solution
|
- Outline the principles of refining of metals by the following methods ...
Text Solution
|
- Define the following terms giving an example of each: (i) Associated...
Text Solution
|
- Write the main products of the following reactions: (i) C(6)H(5)N(2)...
Text Solution
|
- Give reasons for the following: (i) Oxygen is a gas but sulpher is a...
Text Solution
|
- What type of colloid is formed when a solid is dispersed in a liquid? ...
Text Solution
|
- Write the IUPAC name of the following compound:
Text Solution
|
- Write the formula of the compound of sulphur which is obtained when co...
Text Solution
|
- Out of which is an example of vinylic halide?
Text Solution
|
- Unit IUPAC norms write the formulae for the following: (a) Tris (eth...
Text Solution
|
- Draw the structures of the following: (a) H(2)S(2)O(8) (b) CIF(3)
Text Solution
|
- Write the name of the cell which is generally used in inverters. Write...
Text Solution
|
- (a) Write the principle of vapour phase refining. (b) Write the role...
Text Solution
|
- Define the following: (a) Anionic detergents (b) Narrow spectrum a...
Text Solution
|
- Write the structures of the monomers used for getting the following po...
Text Solution
|
- (a) Based on the nature of the intermolecular foces, classify solids b...
Text Solution
|
- Write a point of distinction between a metallic solid and an ionic sol...
Text Solution
|
- Describe a comspicuous change observed when (i) a solution of NaCl...
Text Solution
|