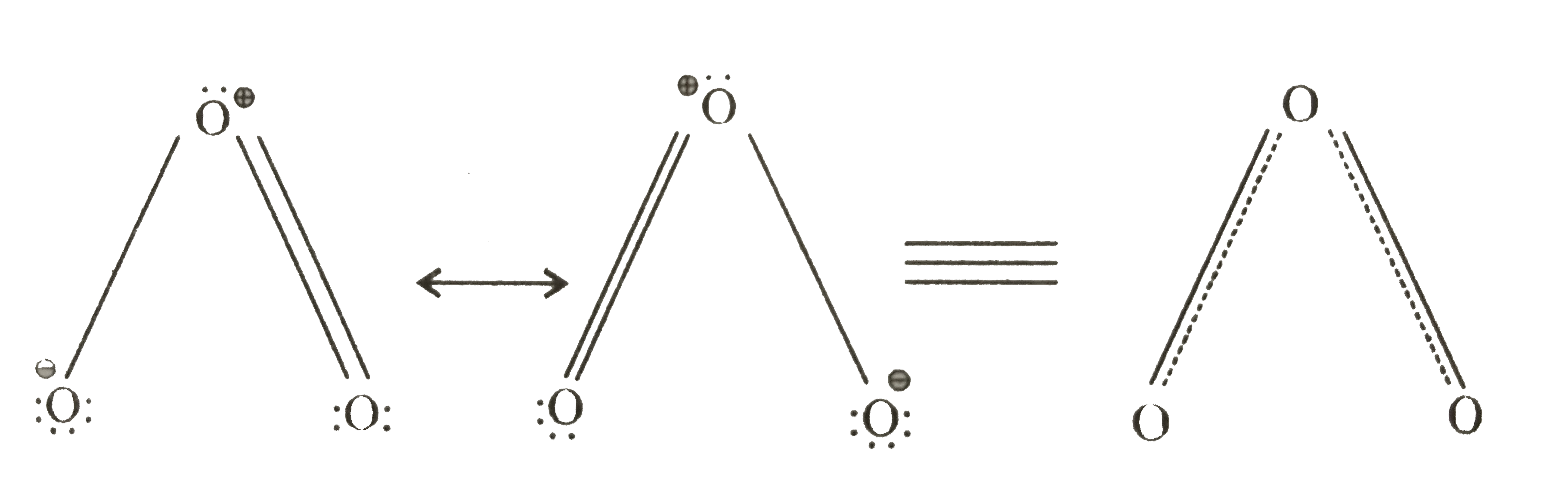
Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise CHEMISTRY (THEORY)|31 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise C.B.S.E CLASS XII|37 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET-II|32 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-SET-III
- Name the principal ore of aluminium. Explain the significance of leach...
Text Solution
|
- Define the following terms with an example in each case: (i) Macromo...
Text Solution
|
- Give reasons for the following : (i) Though nitrogen exhibits + 5 ox...
Text Solution
|
- Write the main products of the following reactions:
Text Solution
|
- , Out of which is an example of a benzylic halide?
Text Solution
|
- Write the formula of the compound of iodine which is obtained when con...
Text Solution
|
- What type of colloid is formed when a gas is dispersed in a liquid? Gi...
Text Solution
|
- Write the IUPAC name of the following compound: CH(3)-O-underset(CH(...
Text Solution
|
- Draw the structure of the following: (a) XeF(4) (b) BrF(5)
Text Solution
|
- Write the name of the cell which is generally used in transistors. Wri...
Text Solution
|
- Using IUPAC norms write the formulae for the following: (a) Potassiu...
Text Solution
|
- (a) What type of isomerism is shown by the complex [Co(NH(3))(5)(SCN)]...
Text Solution
|
- (a) Based on the nature of intermolecular forces, classify the followi...
Text Solution
|
- (a) Write the principle of electrolytic refining. (b) Why does coppe...
Text Solution
|
- Define the following: (a) Cationic detergents (b) Broad spectrum a...
Text Solution
|
- Write the structures of the monomers used for getting the following po...
Text Solution
|
- What is meant by reverse osmosis?.
Text Solution
|
- What type of ores can be concentrated by magnetic separation method ...
Text Solution
|
- Describle the principal controlling each of the following processe...
Text Solution
|
- Explain giving reason: (i) Treansition metals and their compounds g...
Text Solution
|