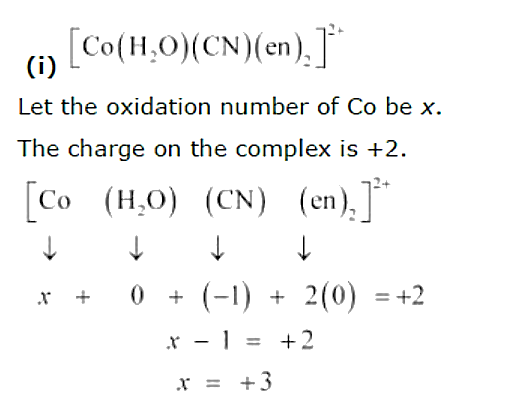Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Set -II|11 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Set -III|11 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise DELHI BOARD SET II|8 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-Set -I
- (a) Which metal in the first transition series (3d series) exhibits +...
Text Solution
|
- Chlorobenzene is extremely less reactive towards nucleophilic substitu...
Text Solution
|
- Explain the mechanism of the reaction is given below : 2CH3-CH2-OHund...
Text Solution
|
- How will you convert (i) Propene to Propan-2-ol? (ii) Phenol to 2...
Text Solution
|
- (a) What type of semiconductor is obtained when silicon is doped with...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 2....
Text Solution
|
- Calculate the emf of the following cell at 298K: Fe(s) abs(Fe^(2+)(0...
Text Solution
|
- How would you account for the following? (l) Transition metals exhib...
Text Solution
|
- Complete the following chemical equations : (i) Cr2O6^(2-)+6Fe^(2+)...
Text Solution
|
- Specify the oxidation number of the metals in the following coordinati...
Text Solution
|
- Given the stuctures of A,B and C in the following reactions- (i) C6...
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- After watching a programme on TV about the adverse effects of junk foo...
Text Solution
|
- (a) Which one of the folJowing is a food preservative? Equanil, Mor...
Text Solution
|
- A reaction is second order in A and first order in B . (i) Write the ...
Text Solution
|
- For a first order reaction, show that time required for 99% completio...
Text Solution
|
- (a) Given reasons for the following : (i) Bond enthalpy of F2 is low...
Text Solution
|
- Account for the following : (i) Helium is used in diving apparatus. ...
Text Solution
|
- How will you bring about the following conversions? (1) Propanone t...
Text Solution
|
- Complete the following reactions : (b) Give simple chemical tests t...
Text Solution
|