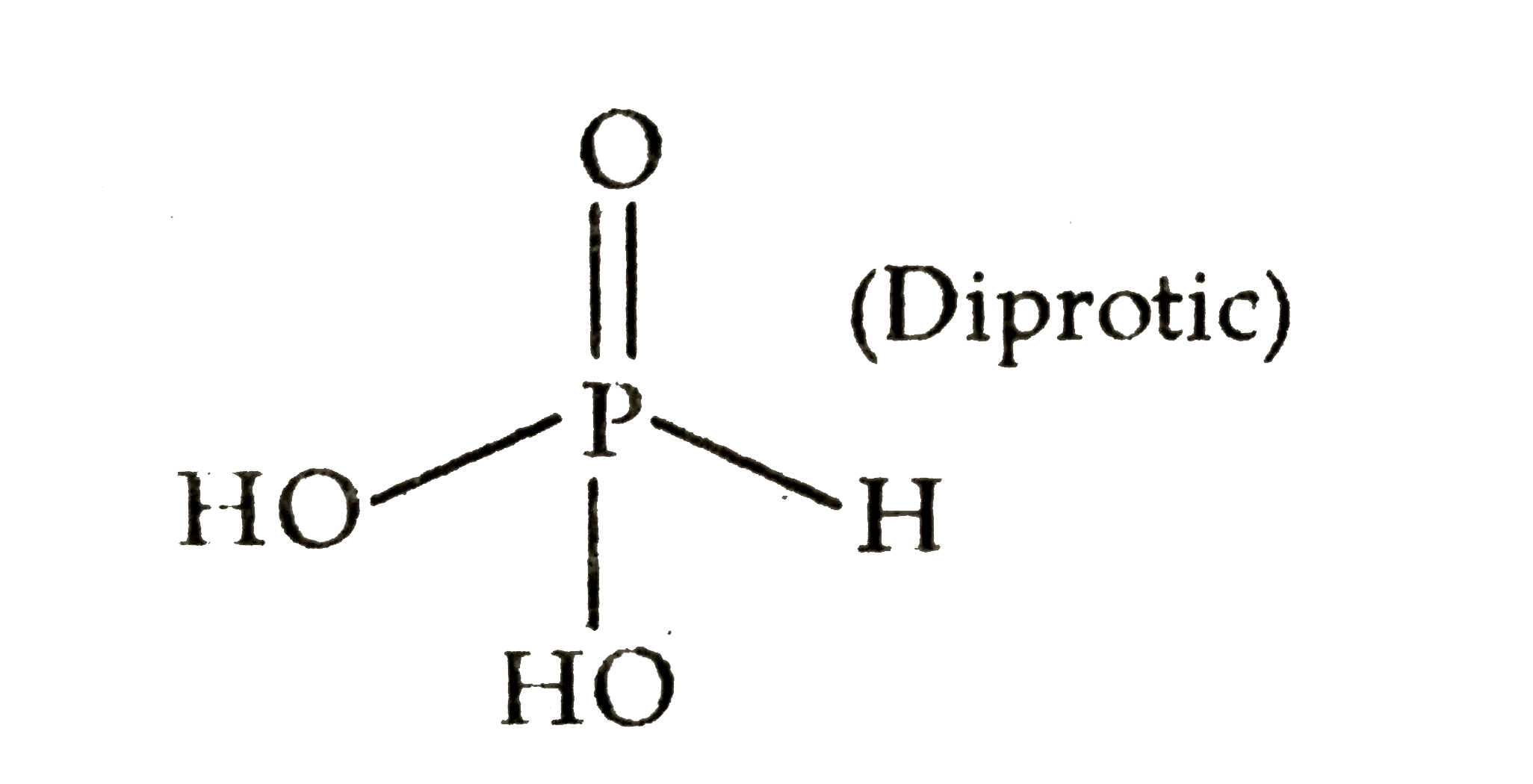Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Questions|29 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise OUTSIDE DELHI (SET-I)|35 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Set - 1 Comptt.|29 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-General Instrucations
- Consider the following reaction : Cu(s)+2Ag^(+)(aq)to 2Ag(s) +Cu^(2+)(...
Text Solution
|
- Write the role of : (i) NaCn in the extraction of gold from its ore....
Text Solution
|
- Complete the following equations : (a) 2MnO(4)^(-)+ 5SO(3)^(2-)+6H^(...
Text Solution
|
- Write the preparation of following : (i) KMnO4 from K2MnO4 (ii) Na...
Text Solution
|
- Do as directed : (i) Arrange the following compounds in the increasi...
Text Solution
|
- Give the formula of monomers involved in the formation of the followin...
Text Solution
|
- Write IUPAC name for each of the following complexes : (i) [Ni(NH3)6...
Text Solution
|
- (i) Complete the following reaction and suggest a suitable mechanism f...
Text Solution
|
- Explain the following : (i) Amino acids behave like salts rather tha...
Text Solution
|
- Write the product(s) formed when (i) 2-Bromopropane undergoes dehydr...
Text Solution
|
- A reaction is first order in A and second order in B (i) Write the d...
Text Solution
|
- Give reason for the following observations : (i) When Silver nitrate...
Text Solution
|
- Calculate the freezing point of an aqueous solution containing 10.5g o...
Text Solution
|
- Mathew works in a multinational company where the working conditions a...
Text Solution
|
- Give simple chemical tests to distinguish between the following pairs ...
Text Solution
|
- (i) Write structure of the product(s) formed : (a) CH3-CH2COOHoverse...
Text Solution
|
- (i) (a) Following is the schematic alignment of magnetic moments : ...
Text Solution
|
- Silver metal crystallises with a face centred cubic lattice. The lengt...
Text Solution
|
- What happens when (a) Chlorine gas reacts with cold and dilute solut...
Text Solution
|
- Complete the following reactiosn: (a) Cu+HNO3("dilute")to (b) Fe^(...
Text Solution
|