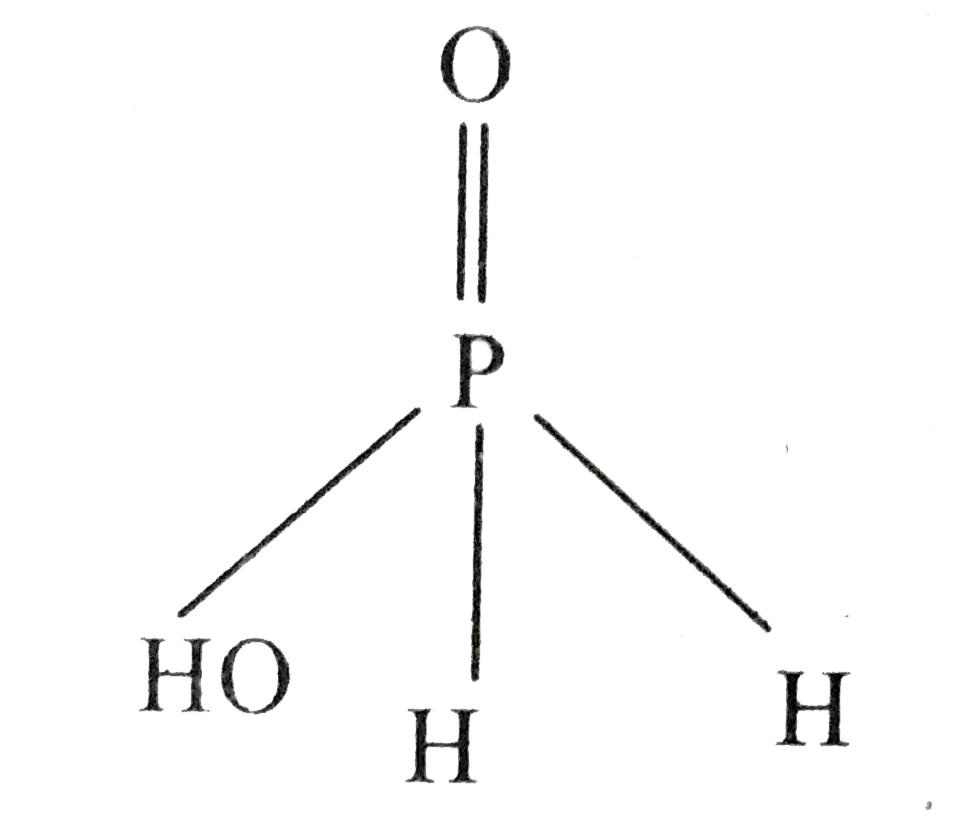
Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Outside Delhi : SET-III|11 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION-A|13 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise [SET-III]|10 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-Outside Delhi : SET-II
- Which stoichiometric defect in crystals increses the density a solid?
Text Solution
|
- What is shape - selective catalysis ?
Text Solution
|
- What is the role of collectors in Froth Floatation process ?
Text Solution
|
- Write the IUPAC name of PH-CH=CH-CHO.
Text Solution
|
- Explain the cleaning action of soap . Why do soaps not work in hard wa...
Text Solution
|
- A Voltaic cell is set up at 25^(@)C with the following half cells ?
Text Solution
|
- Explain the following observations : (i) Many of the transition elem...
Text Solution
|
- Explain the following giving one suitable example in each case (i) E...
Text Solution
|
- Explain the following observations : (i) Nitrogen is much less react...
Text Solution
|
- (a) Draw the structures of the following molecules : (i) N(2)O(5) ...
Text Solution
|