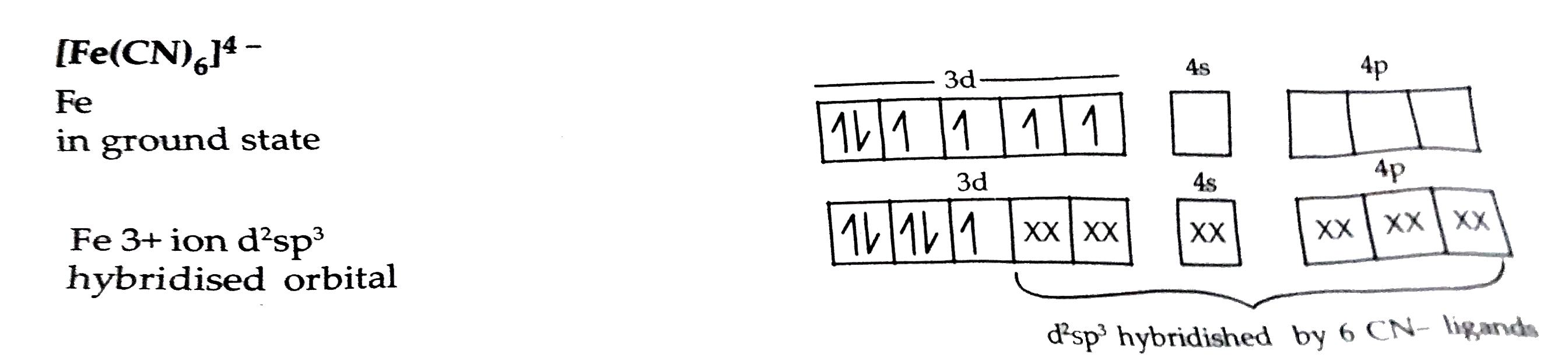Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise [SET-1]|36 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Outside Delhi: Set-II|11 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise [OUTSIDE DELHI : SET -III]|10 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-SET-1
- Define rate constant (k) ?
Text Solution
|
- Why is Tyndall effect shown by colloidal solutions ?
Text Solution
|
- Write the IUPAC name of the following coordination compound [NiCl(4)]^...
Text Solution
|
- Out of CH(3)OH and phenol (##SBCHMXIIODI2016E01004Q01.png" width="80%...
Text Solution
|
- What are Associated Colloids ? Given an example.
Text Solution
|
- Explain the following terms with subitable examples : {:((i),"Frenke...
Text Solution
|
- Define osmotic pressure of a solution. How is the osmotic pressure ore...
Text Solution
|
- What happens when (i) Concentrated H2 SO4 is added to calcium fluori...
Text Solution
|
- Given reasons : (i) Zn is not regarded as a transition element. (ii...
Text Solution
|
- Write the equations for the preparation of 1-bromobutane from : (i) ...
Text Solution
|
- Which compound in the following pairs will react faster in SN^(2) reac...
Text Solution
|
- Silver crystalilises in a fcc lattice. The edge length of its unit is ...
Text Solution
|
- An aqueous solution of 2 per cent (wt.//wt) non-volatile solute exerts...
Text Solution
|
- The rates of most reaction double when their temperature is raised fro...
Text Solution
|
- Explain the following terms : (i) "Peptization"" "(ii)"Loyphobic co...
Text Solution
|
- Outline the principles of refining of metals by the following methods ...
Text Solution
|
- Describe the preparation of potassium permanganate. How does the acidi...
Text Solution
|
- What is lanthanoid contraction? What are the consequences of lanthanol...
Text Solution
|
- Writen the hybridization, shape and magnetic character of [Fe(CN)(6)]^...
Text Solution
|
- What happpens when : (i) CH(3)-CI is treated with aqueous KOH? (ii...
Text Solution
|