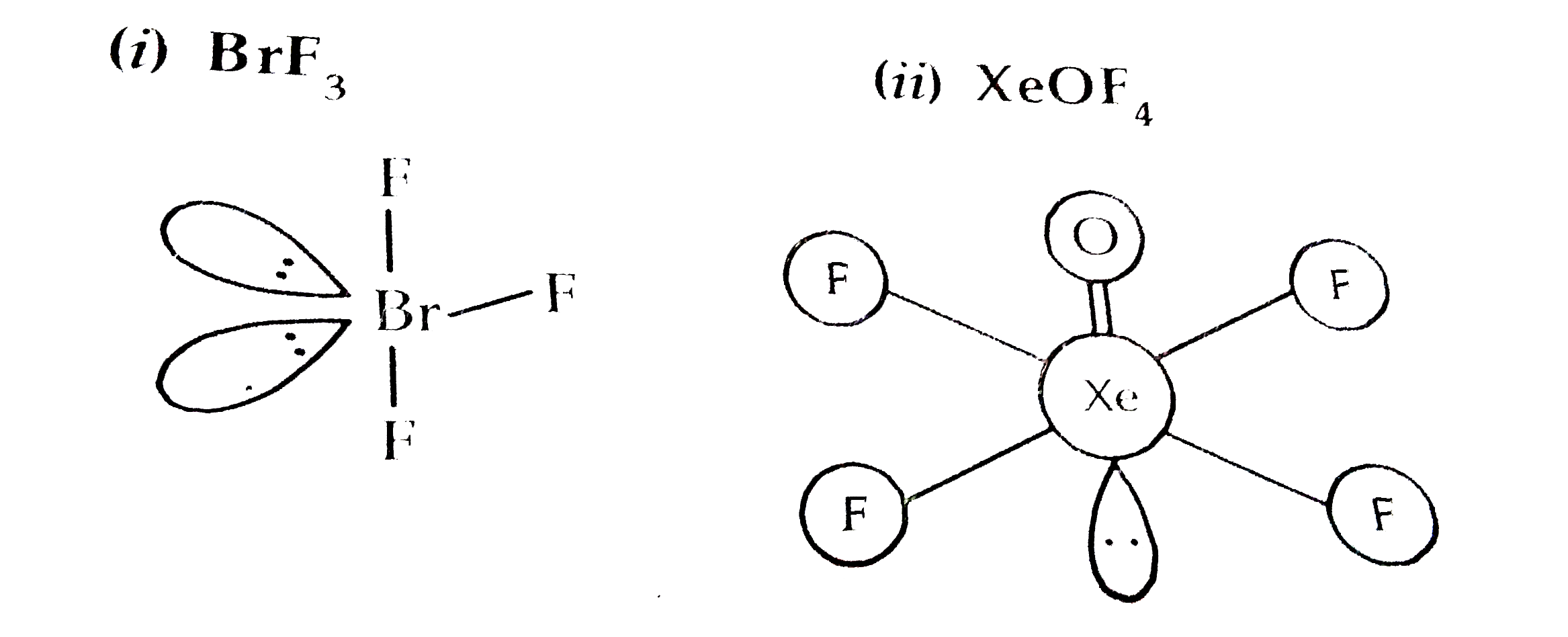Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise [SET-II]|10 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise [SET-III]|10 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise Delhi Board : Set - III|7 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-[SET-I]
- Classify the following as primary ,secondary and tertiary alcohols : ...
Text Solution
|
- How would you account for the following : (i) The metallic radii of ...
Text Solution
|
- Complete the following reaction equations: (i) R-overset(O)over...
Text Solution
|
- Describe the following substance with one suitable expample of each t...
Text Solution
|
- (a) Define the following terms: (i) Mole fraction (ii) Van't Hoff fa...
Text Solution
|
- (a) What is meant by: (i) Colligative properties (ii)Molality of a sol...
Text Solution
|
- Draw the structures of the following : (i) H(2)S(2)O(8) (ii...
Text Solution
|
- Explain the following observation : In the structure of HNO(3) ...
Text Solution
|
- Write chemical equations to illustrate the following name bearing reac...
Text Solution
|
- How will you bring about the following conversions : (i) Ethanol...
Text Solution
|
- Explain the following with an exampe in each : {:((i)"Kolbe's reacti...
Text Solution
|
- Write the products A and B in the following :
Text Solution
|
- Write two uses of each of the following polymers. "(i) Polypropylene...
Text Solution
|
- What are enzyme ? Describe their functions. Name two diseases which ar...
Text Solution
|
- Ankit's grandfather is not only obses but he is also a diabetic patien...
Text Solution
|
- (a) What are the two classifications of batteries ? What is the differ...
Text Solution
|
- (a) What are fuel cells ? Give an example of a fuel cell. (b) Calcu...
Text Solution
|
- Draw the structure of : {:((i)BrF(3),(ii)XeOF(4)):} (b) Explain givi...
Text Solution
|
- (a) (i) Why PCl(5) gives fumes in moisture ? (ii) Why Interhalogens ...
Text Solution
|
- (a) What is meant by the following terms ? Give an example of the reac...
Text Solution
|