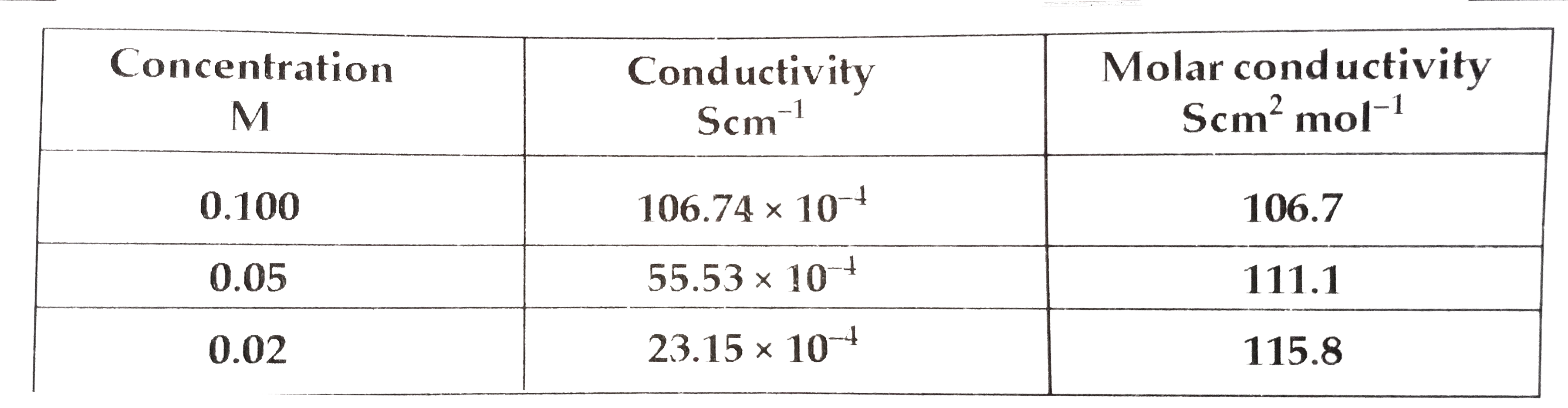Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET- I|34 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET- II|10 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise QUESTION PAPER (SECTION-B)|10 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-QUESTION PAPER (SECTION-C)
- Give reasons for the following : (a) When 2g of benzoic acid is diss...
Text Solution
|
- An alcohol [A] with molecules formula (C(4)H(10)O) o oxidation with ac...
Text Solution
|
- Which one of the following compounds will undergo hydrolysis at a fast...
Text Solution
|
- A compound is formed by the substitution of two chlorine atoms for two...
Text Solution
|
- Give reasons for the following : (i) Use of aspartame as an artifici...
Text Solution
|
- (a) Name the branched chain component of starch. (b) Ribose in RNA a...
Text Solution
|
- Give three reactions of glucose which cannot be explained by its open ...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- Two reactions of the same order have equal pre exponential factors but...
Text Solution
|
- (a) A colloidal sol is prepared by the given method in figure. What is...
Text Solution
|
- Describe how the following steps can be carried out? (a) Recovery o...
Text Solution
|
- Explain the use of the following : (a) NaCN in Froth Floatation Method...
Text Solution
|
- Explain the following : (a) Out of Sc^(3+), Co^(2+) " and "Cr^(3+) "...
Text Solution
|
- A metal complex having composition Cr(NH(3))(4)CI(2)Br has been isolat...
Text Solution
|
- (a) Identify A-D (b) Distinguish between the following pair of compo...
Text Solution
|
- (a) Account for the following : (i) Direct nitration of aniline yie...
Text Solution
|
- (a) A cell is prepared by dipping a zinc rod in 1M zinc sulphate solut...
Text Solution
|
- (a) Apply Kohlrausch law of independent migration of ions, write the e...
Text Solution
|
- (a) Account for the following observations : (i) SF(4) is easily hyd...
Text Solution
|
- (a) What inspired N.Bartlett for carrying out reaction between Xe and ...
Text Solution
|