Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
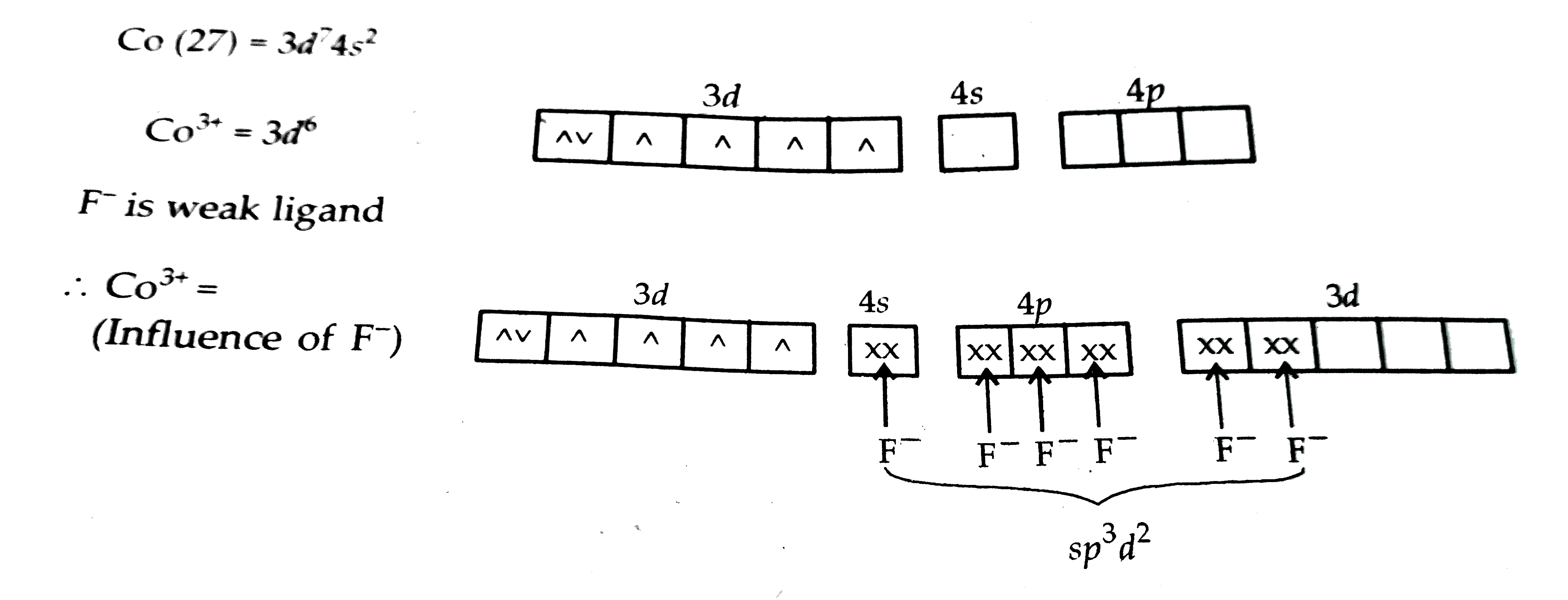
- For the complexion [CoF(6)]^(3-) write the hybridization type, magneti...
Text Solution
|
- K(3)CoF(6) is high spin complex. What is the hybrid state of Co atom i...
Text Solution
|
- For the complex , [Co(en)(3)]^(3+) , write the hybridisation type, mag...
Text Solution
|
- For the complexion [CoF(6)]^(3-) write the hybridization type, magneti...
Text Solution
|
- (i) What type of isomerism is shown by the complex [Cr(H(2)O)(6)]Cl(3)...
Text Solution
|
- [CoF(6)]^(3-) संकुल आयन में अयुग्मित इलेक्ट्रॉनों की संख्या होगी (Co क...
Text Solution
|
- For the complexion [CoF(6)]^(3-) write the hybridization type, magneti...
Text Solution
|
- संकुल [CoF(6)]^(3-) में सकरण और अयुग्मिक इलेट्रॉनों की संख्या लिखिए। (...
Text Solution
|
- कॉम्प्लेक्स [CoF(6)]^(3-) के लिए संकरण प्रकार, चुंबकीय व्यवहार और स्पि...
Text Solution
|