Text Solution
Verified by Experts
Topper's Solved these Questions
XII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET- II|10 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SET- III|10 VideosXII BOARDS
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise QUESTION PAPER (SECTION-C)|21 VideosSAMPLE PAPER 2019
XII BOARD PREVIOUS YEAR PAPER ENGLISH|Exercise SECTION: D|1 Videos
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-SET- I
- How would you obtain (i). Picric acid (2, 4, 6-trinitrophenol) from...
Text Solution
|
- What is essentially the difference between alpha-form of glucose and b...
Text Solution
|
- Describe what do you understand by the primary and secondary structure...
Text Solution
|
- Arrange the following polymers in increasing order of their intermolec...
Text Solution
|
- Silver crystallizes in face-centred cubic unit cell. Each side of this...
Text Solution
|
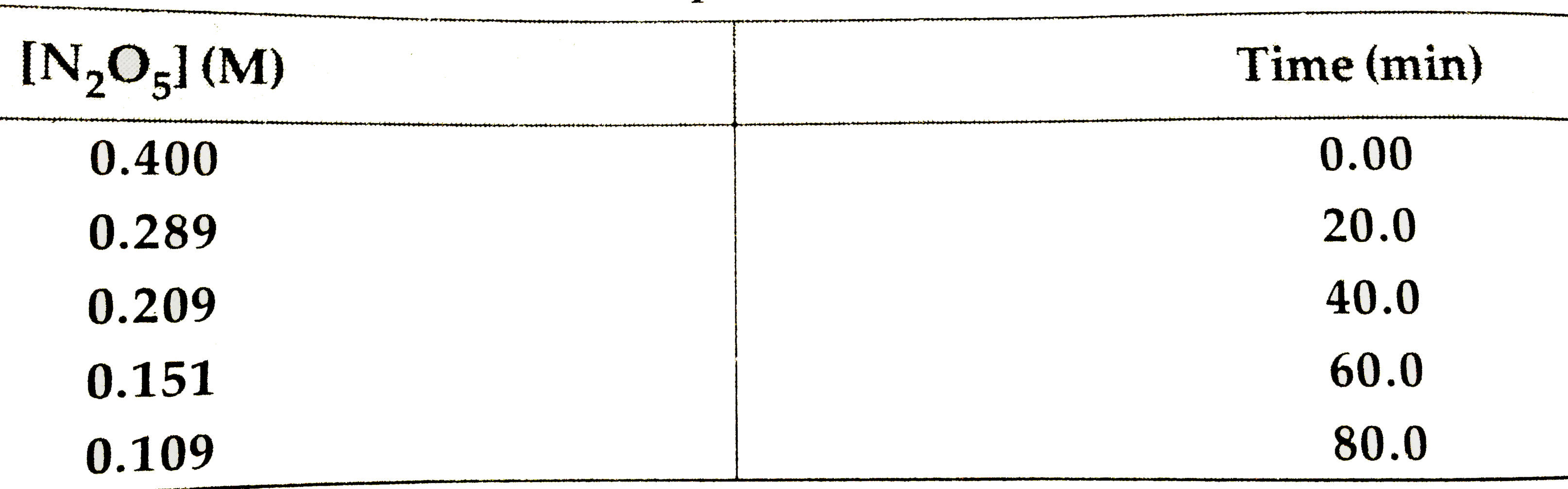
- Nitrogen pentoxide decomposes according to equation : 2N2O5(g)to4NO...
Text Solution
|
- Explain clearly how the phenomenon of adsorption finds applications in...
Text Solution
|
- What are the different types of RNA found in the cell?
Text Solution
|
- Describe the principle behind each of the following processes : (i)...
Text Solution
|
- Complete the following chemical equations: (i) Na2CrO4+H2SO4to (ii...
Text Solution
|
- Write the name, the structure and the magnetic behaviour of each one o...
Text Solution
|
- Answer the following: (i) Haloalkanes easily dissolve in organic so...
Text Solution
|
- Name the following compounds according to IUPAC system. (i) CH3-un...
Text Solution
|
- Describe the foJlowing giving one example for each : (i) Detergents...
Text Solution
|
- (a) Differentiate between molarity and molality for a solution. How do...
Text Solution
|
- (a) Define the terms osmosis and osmotic pressure. Is the osmotic pres...
Text Solution
|
- A translucent white waxy solid (A) on heating in an inert atomosphere ...
Text Solution
|
- (a) What is meant by unidentate, bidentate and ambidentate ligands? Gi...
Text Solution
|
- (a) Explain the following : (i) NF(3) is an exothermic compound wher...
Text Solution
|
- (a)Account for the following : (i)The acidic strength decreases in t...
Text Solution
|