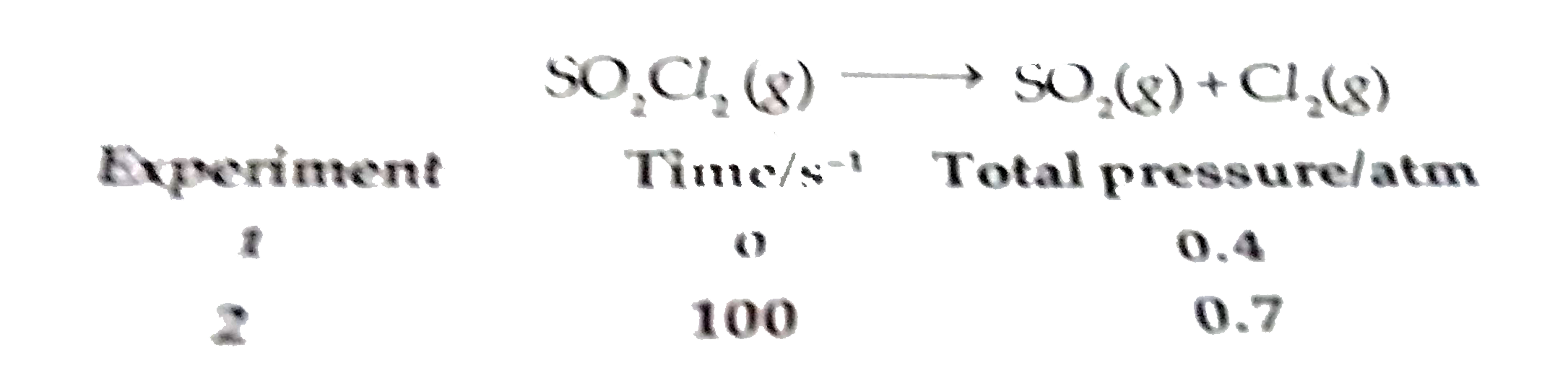Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARD PREVIOUS YEAR PAPER ENGLISH-XII BOARDS-C.B.S.E. CLASS-XII
- Draw the structures of the following molecules : (i) XeOF(4) ...
Text Solution
|
- Account for the following : (i) PCl(5) is more covalent than PCl(3)...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- (i) Give two examples of macromolecules that are chosen as drug target...
Text Solution
|
- (i) Deficiency of which vitamin causes night-blindness ? (ii) Name t...
Text Solution
|
- After the ban on plastic bags, students of one school decided to make ...
Text Solution
|
- (A)Write the mechanism of the following raction : CH(3)CH(2)OH overs...
Text Solution
|
- Given the structures of A, B and C in the following reactions : (i)...
Text Solution
|
- How will you canvert the following : (i) Nitrobenzene into aniline, ...
Text Solution
|
- (a) Define the following terms : (i) Limiting molar conductivity, ...
Text Solution
|
- State Faraday's first law of electrolysis . How much charge in terms o...
Text Solution
|
- How do you prepare : (i) k(2)MnO(4) from MnO(2)? (ii) Na(2)Cr(2)O...
Text Solution
|
- (i) Name the element of 3d transition series which shows maximum numbe...
Text Solution
|
- Account for the following : (i) CH(3)CHO is more reactive than CH(3)...
Text Solution
|
- Give one example each of sol and gel.
Text Solution
|
- Some liquids on mixing from 'azeotropes'. What are 'azeotropes'?
Text Solution
|
- Which component of starch is a branched polymer of alpha-glucose and i...
Text Solution
|
- State Henry's law. What is the effect of temperature on the solubility...
Text Solution
|
- Define the following terms : (i) Pseudo first order reactions (ii...
Text Solution
|
- Write the principle behind the following methods of refining : (i)Hy...
Text Solution
|