Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) In the formation of compound between two atoms A and B, A loses ...
Text Solution
|
- An atom of an element A has three electron in its outer shell and B ha...
Text Solution
|
- If the electron pair forming a bond between two atoms and B is not in ...
Text Solution
|
- Atom A,B and C occur in the same period and have one, six and seven va...
Text Solution
|
- An element A has an atomic number of 6. Another element B has 17 elect...
Text Solution
|
- In the formation of the compound AB, atoms of A lost one electron each...
Text Solution
|
- What is the name of the chemical bond formed: (a). By the sharing of e...
Text Solution
|
- The electronic configurations of two elements A and B are given below:...
Text Solution
|
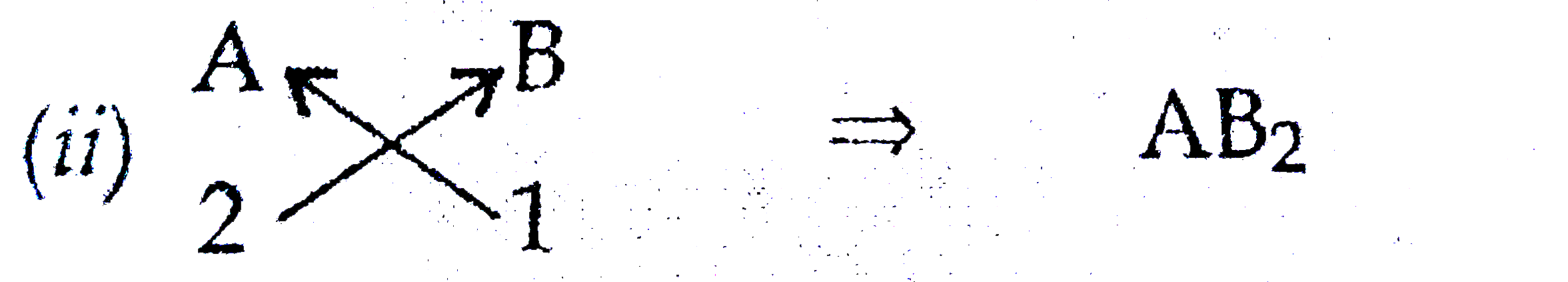
- (a) in the formation of compound between two atoms A and B, loses two ...
Text Solution
|