Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
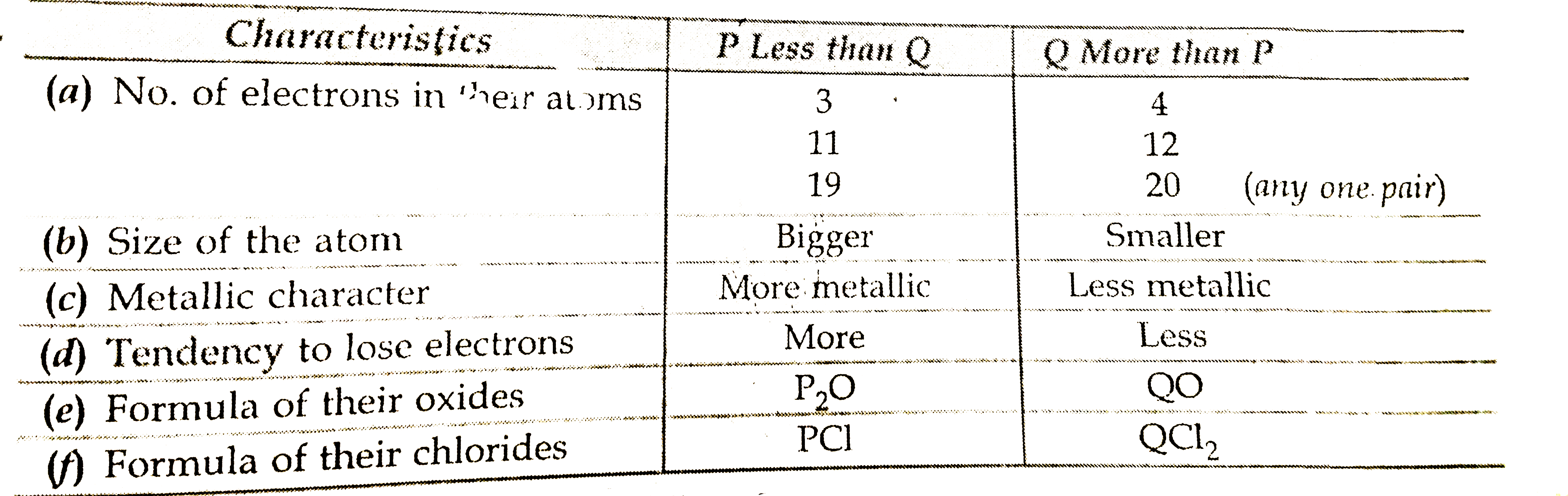
- Tow elelments 'I' and 'Q' belong to the same periiod of the modern per...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 respectively in the same ...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 respectively in the same ...
Text Solution
|
- Two elements 'P' and 'Q' belong to the same period of the modern perio...
Text Solution
|
- Two elements X and Y belong to groups 1 and 2 respectively in the same...
Text Solution
|
- दो तत्वों A तथा B आधुनिक आवर्त सारणी के तीसरे आवर्त और क्रमशः वर्ग 2 ए...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements M and N beong to group I and II respectively and are in t...
Text Solution
|