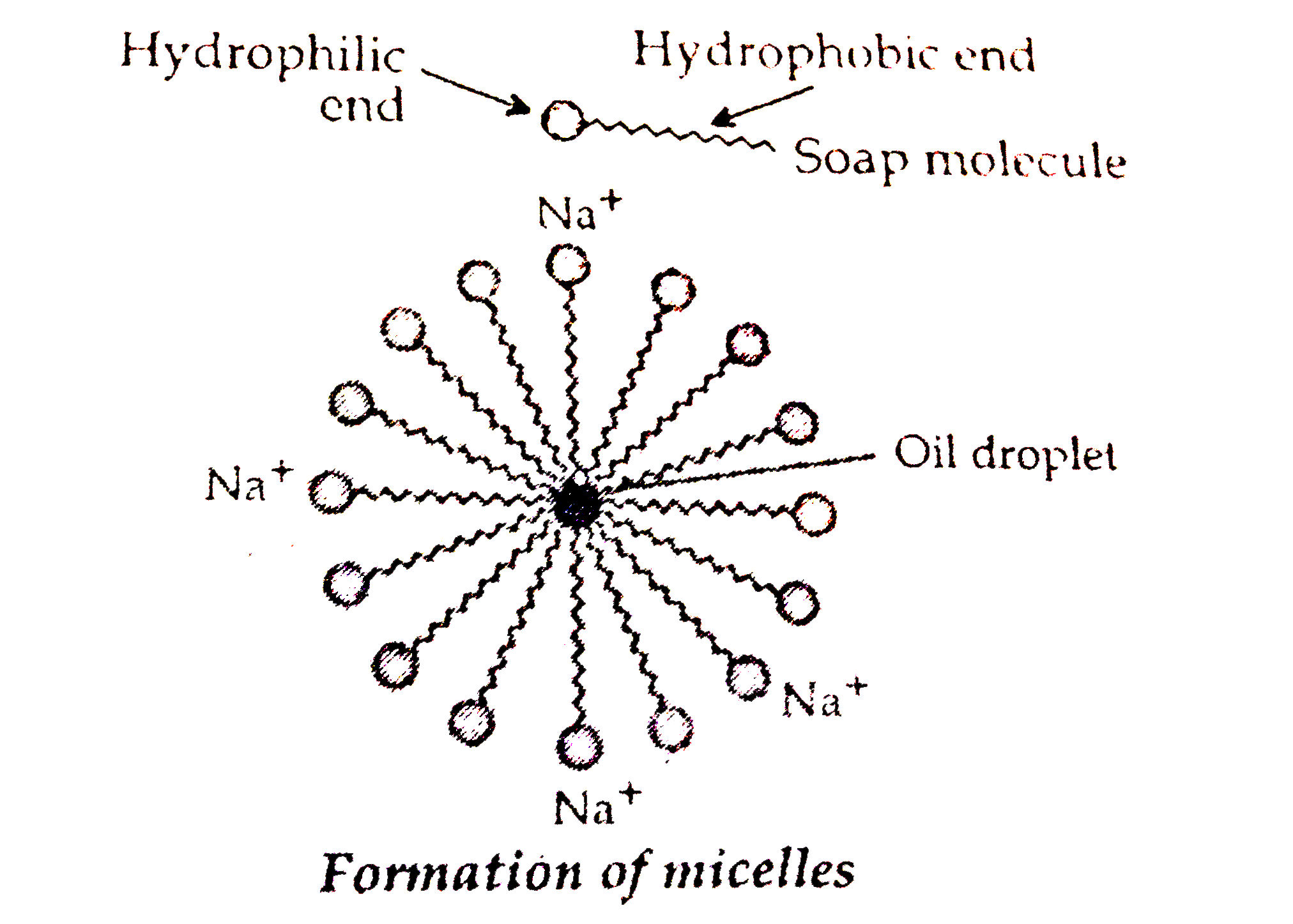
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Both soap and detergent are some type of salts. What is the difference...
Text Solution
|
- Both soap and detergent are some type of salts. What is the difference...
Text Solution
|
- What is the difference between soaps and detergements ? State in b...
Text Solution
|
- Describe the cleansing action of a soap and a detergent.
Text Solution
|
- Explain the mechanism of cleansing action of soaps and detergents.
Text Solution
|
- Explain the mechanism of cleansing action of soaps and detergents.
Text Solution
|
- Why hard water does not form lather with soap ? What advantage has soa...
Text Solution
|
- Explain the mechanism of cleansing action of soaps and detergents.
Text Solution
|
- Explain the mechanism of cleansing action of soaps and detergents. Mec...
Text Solution
|