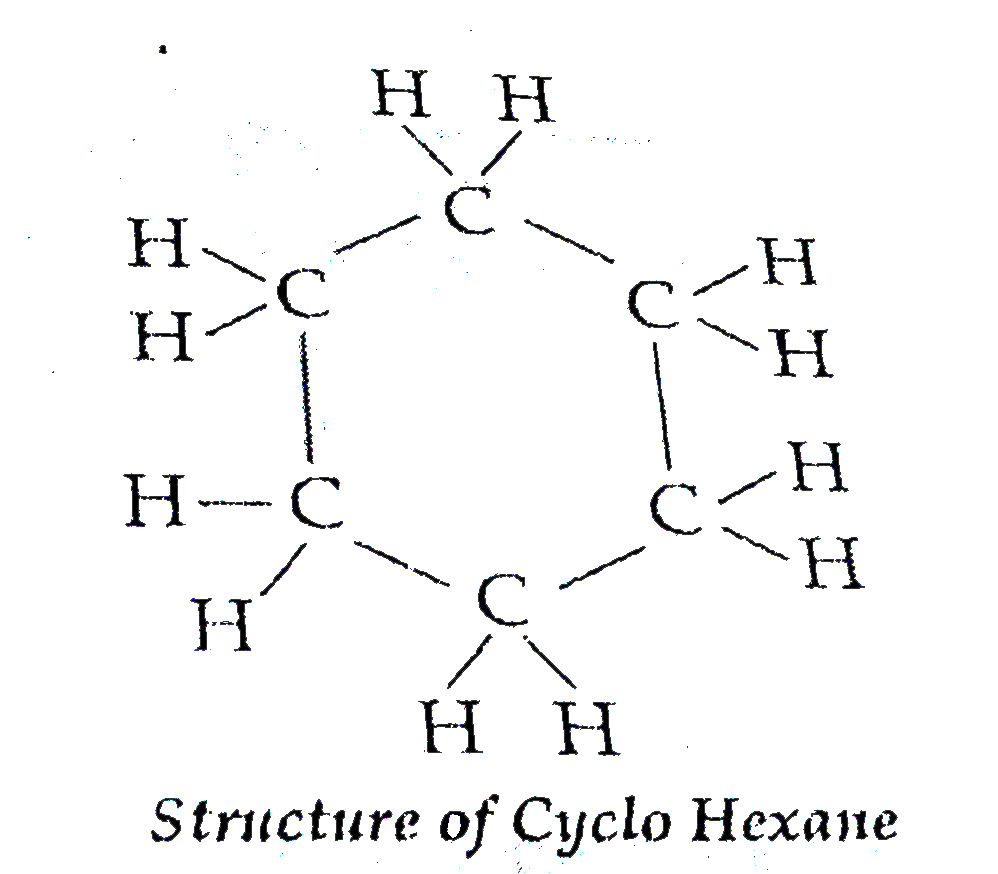
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Why are most carbon compounds poor conductors of electricity ? ...
Text Solution
|
- (a) Why are most carbon compounds poor conductors of electricity? (b...
Text Solution
|
- (a) Why are most carbon compounds poor conductors of electricity ? (b)...
Text Solution
|
- Carbon compounds in which the carbon atoms are linked to each other by...
Text Solution
|
- Carbon forms a large number of compounds because of its two unique pro...
Text Solution
|
- Carbon forms a large number of compounds because of its two unique pro...
Text Solution
|
- Carbon forms a large number of compounds because of its two unique pro...
Text Solution
|
- Carbon forms a large number of compounds because of its two unique pro...
Text Solution
|
- Carbon compounds in which the carbon atoms are linked to each other by...
Text Solution
|