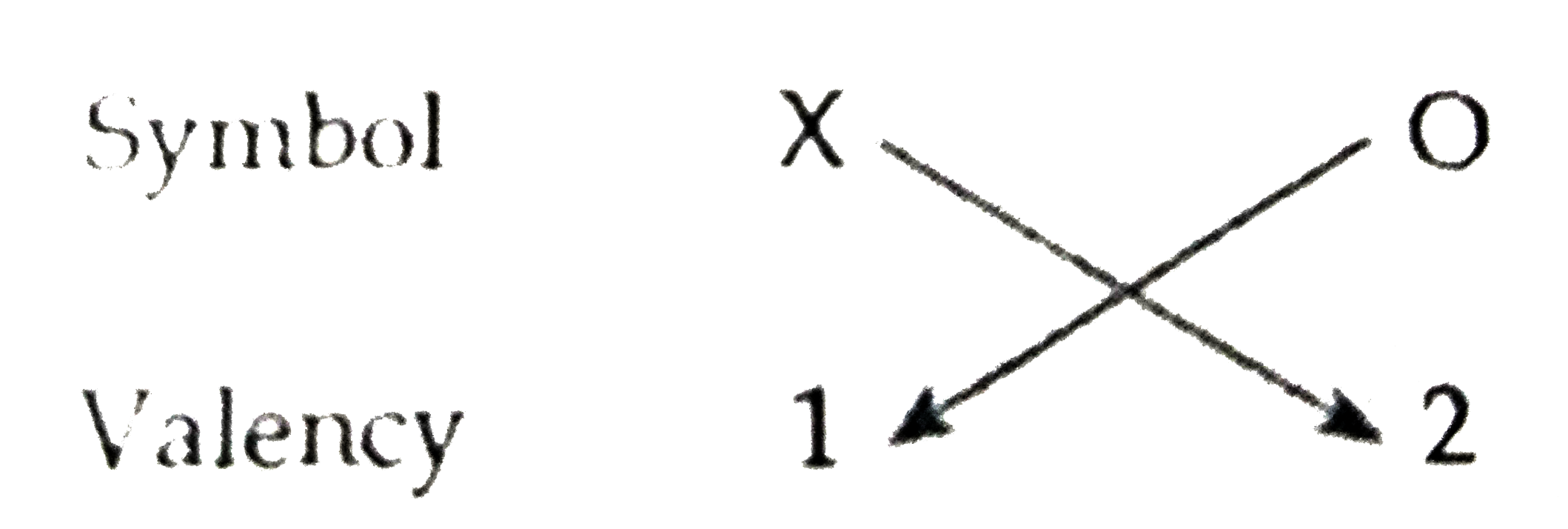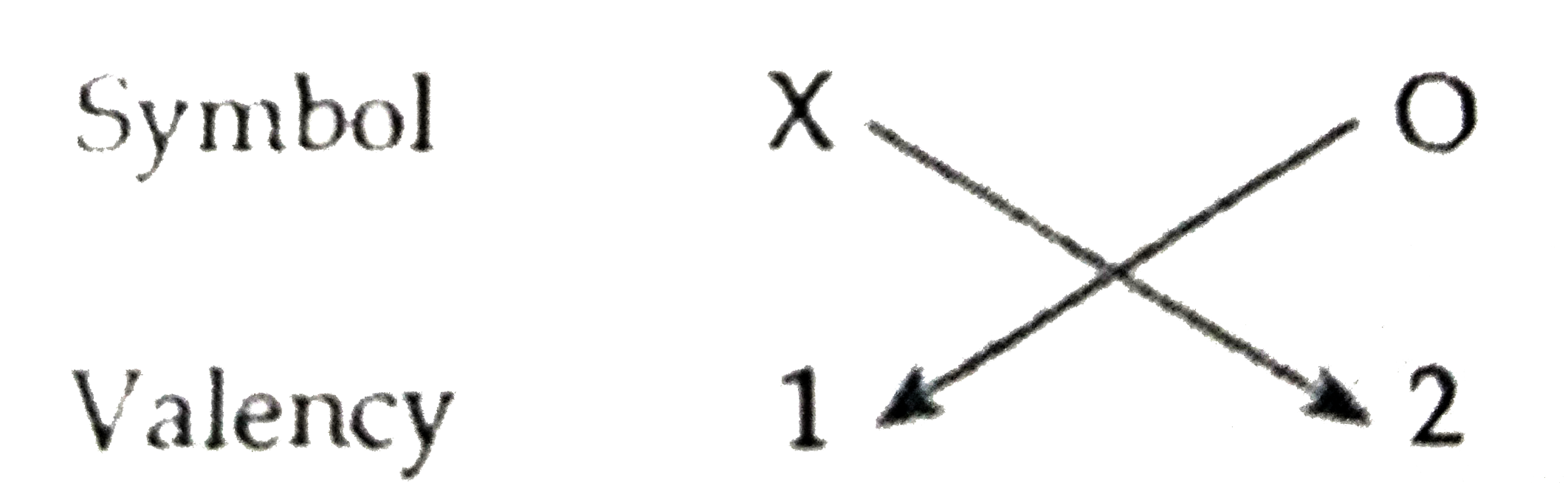Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Based on the group valency of elements write the molecular formula of ...
Text Solution
|
- Based on the group valency of elements state the formula for the follo...
Text Solution
|
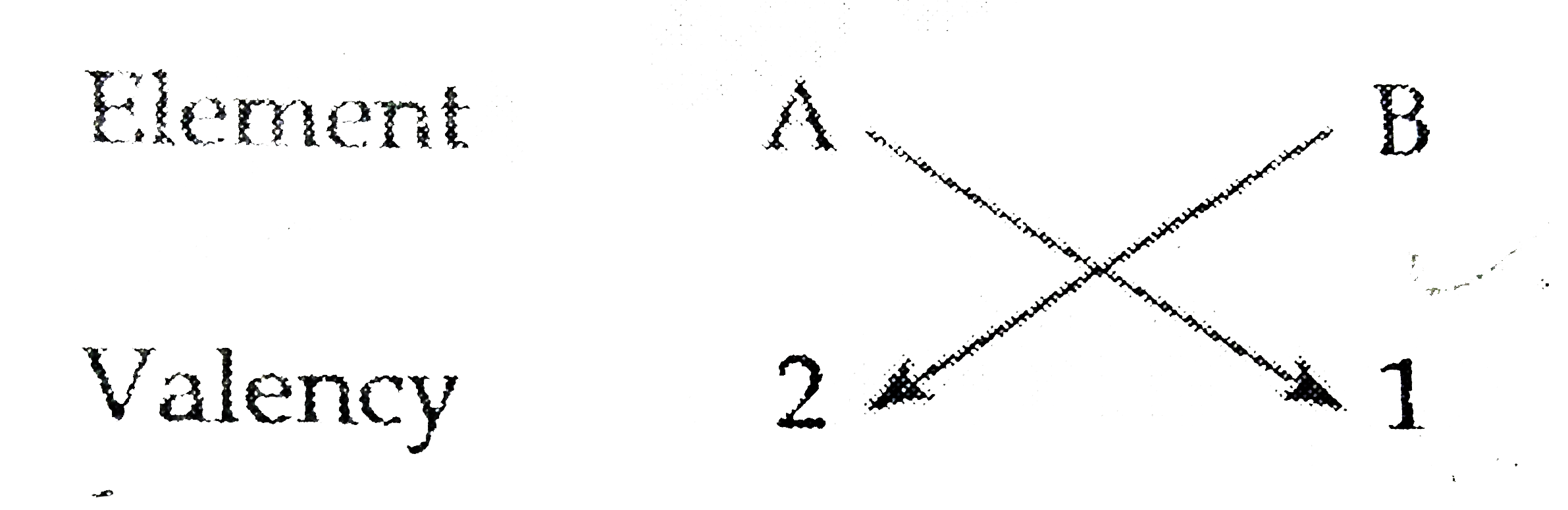
- Based on the group valenvy of elements write the molecular formula of ...
Text Solution
|
- एक तत्व का इलेक्ट्रॉनिक विन्यास 2,8,1 है । (i)इस तत्व का नाम लिखिए...
Text Solution
|
- Element A of group VA combines with element B of group VI A. The resul...
Text Solution
|
- Element A of group VA combines with element B of group VI A. The resul...
Text Solution
|
- What is the formula of binary compound formed between : 2nd element of...
Text Solution
|
- Based on the group valency of elements state the formula for the follo...
Text Solution
|
- Based on the group valency of elements state the formula for the follo...
Text Solution
|