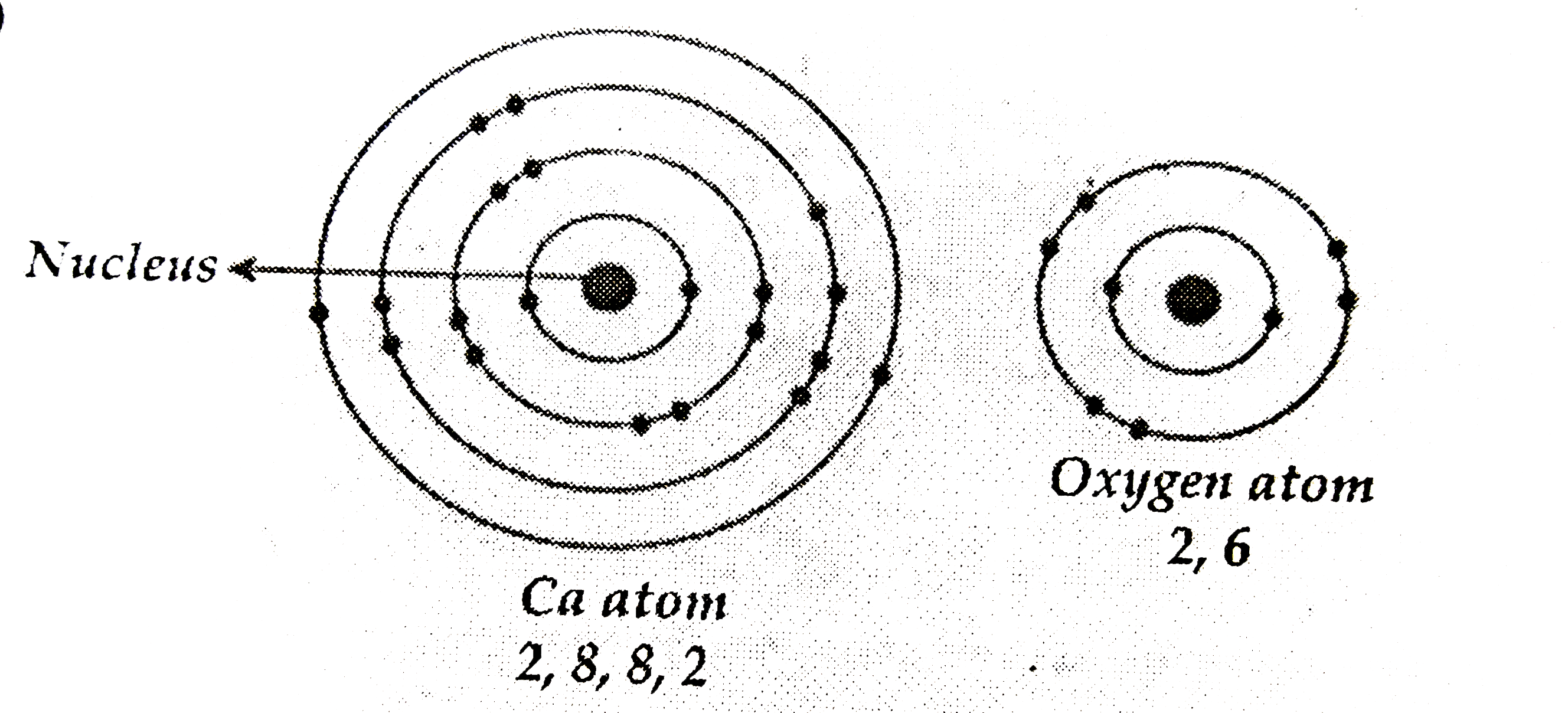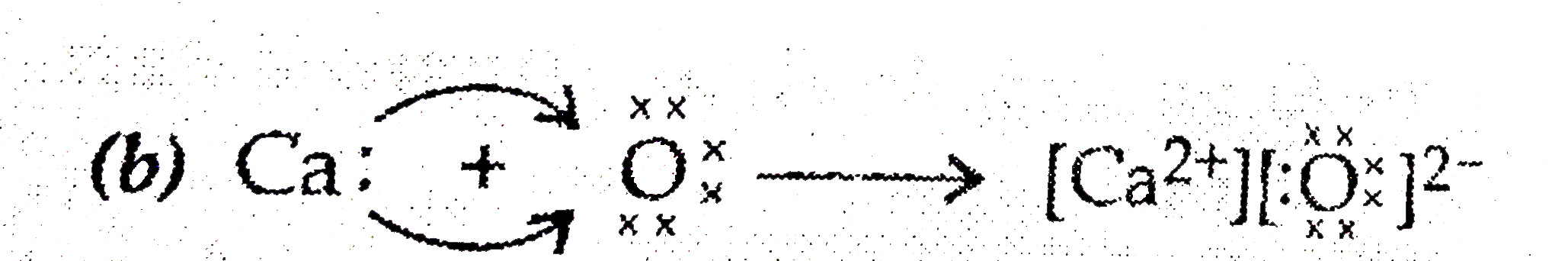Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Write the electron dot structure for calcium and oxygen. The ato...
Text Solution
|
- The weight of calcium oxide formed by burning 20 g of calcium in exces...
Text Solution
|
- (a) Write the electron dot structure for calcium and oxygen. The atomi...
Text Solution
|
- a. Write electron dot diagram for chlorine (At No. 17) and Calcium (At...
Text Solution
|
- (i) Explain the formation of ionic compound CaO with electron dot stru...
Text Solution
|
- a. Explain the formation of Calcium Chloride with the help of electron...
Text Solution
|
- (i) Write the electron-dot structures for sodium, oxygen and magnesium...
Text Solution
|
- Explain the formation of ionic compound CaO with electron-dot structur...
Text Solution
|
- (i) Explain the formation of ionic compound CaO with electron dot stru...
Text Solution
|