Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Using the above table answer the following questions : (a) Compare t...
Text Solution
|
- Use the periodic table to answer the following questions. a. Identif...
Text Solution
|
- Atomic number of few elements are given below 10, 20, 7, 14 (a) Identi...
Text Solution
|
- The following diagram shows a part of the periodic table containing fi...
Text Solution
|
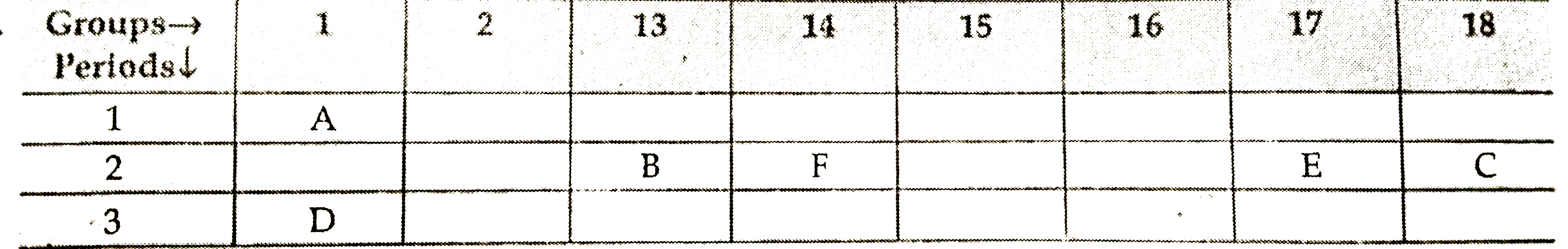
- The following table shows the position of six elements A, B, C, D, E a...
Text Solution
|
- Using the above table answer the following questions : (a) Compare the...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- Use the periodic table to answer the following questions (a) Identify ...
Text Solution
|
- Answer these key questions using the periodic table section above. A) ...
Text Solution
|